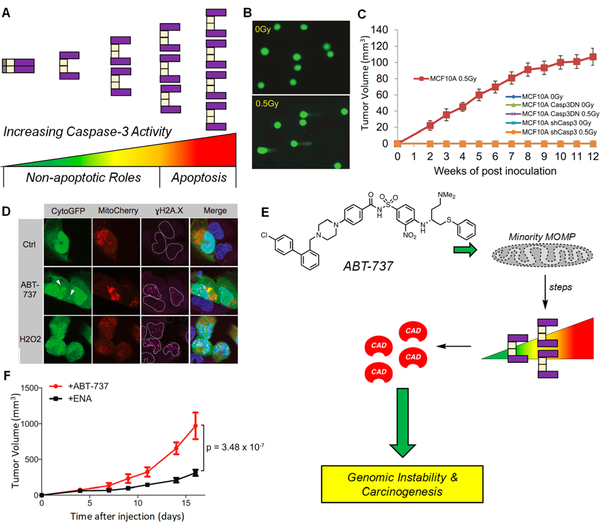
Figure 6.
Promotion of DNA damage and genomic instability by subapoptotic caspase-3 activity. (A) Graphical representation of the spectrum of PC-3/caspase-3 activity. Sublethal levels (spanning from green to yellow/orange) represent proteolytic levels not sufficient for apoptotic induction, but sufficient for cleavage of a variety nonlethal protein substrates. Lethal levels (spanning from orange to red) signify irreconcilable caspase-3 activities and result in cell death via apoptosis. (B) Sublethal irradiation of MCF10A induces DNA damage as per the comet tail assay. A larger tail moment indicates increased DNA damage. Reprinted with permission from ref 142. Copyright 2015 Elsevier. (C) Irradiated MCF10A cells form tumors in mice, while shRNA of CASP3 leads to a dramatic reduction in tumor formation (n = 10 per arm, all arms are displayed in the panel). These results indicate an active role of caspase-3 in tumorigenesis. Casp3DN: dominant-negative caspase-3. Reprinted with permission from ref 142. Copyright 2015 Elsevier. (D) Treatment of U20S cells, transiently expressing CytoGFP/Mitocherry, with ABT-737 (5 μΜ) leading to increased /H2AX foci; H202 is the positive control. Increases in yH2AX foci are indicative of heightened DNA damage. Reprinted with permission from ref 145. Copyright 2015 Elsevier. (E) Treatment with ABT-737 causes minority MOMP (displayed as a perforated mitochondria), leading to sublethal levels of caspase-3, resulting in CAD activation and genomic instability and carcinogenesis. (F) Primary pi9Arf null MEF cells were treated with ABT-737 (10 μΜ) or its inactive enantiomer (ENA, 10 μΜ) for 10 passages, then inoculated into mice. Tumor formation is increased when cells were pretreated with ABT-737 (n = 15 per treatment), suggesting that ABT-737 treatment leads to caspase-3 mediated genomic instability that ultimately increases tumorigenicity. Adapted with permission from ref 145. Copyright 2015 Elsevier.