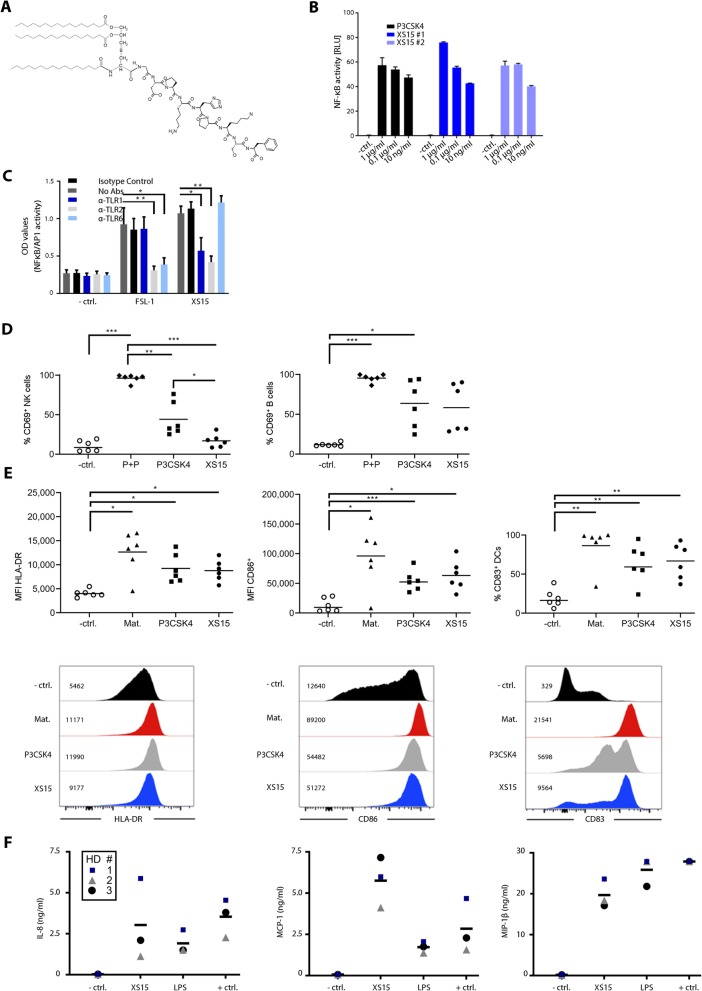
Fig. 1.
Pam3Cys-GDPKHPKSF (XS15) is a TLR1/2 ligand activating immune cells and stimulating DCs and cytokine release. (a) Structure of Pam3Cys-GDPKHPKSF: Skeletal structural formula of the molecular structure of the lipopeptide Pam3Cys-GDPKHPKSF termed XS15. (b) Dual-luciferase assay on HEK293T cells transfected with TLR2: HEK293T cells were transiently transfected with a human TLR2 plasmid and a NF-κB luciferase reporter plasmid or left untreated (− ctrl.). Culture medium was replaced after 30 h and stimuli added at the stated concentrations. The cells were incubated for 18 h and lysates were prepared and analysed by dual-luciferase assay. Pam3CysSK4 (P3CSK4) and two different lots of XS15 (XS15#1/ XS15#2) were used. (c) HEK-Dual hTLR2 cells, stably expressing a NF-κB/AP-1-inducible secreted embryonic alkaline phosphatase (SEAP) reporter, were incubated for 1 h with TLR1, TLR2 and TLR6 blocking antibodies, isotype control or negative controls (no Abs) (4 μg/ml). Then, cells were stimulated for 24 h with the established TLR2/6 agonist FSL-1 (1 ng/ml), XS15 (10 ng/ml) or left unstimulated (− ctrl.). Supernatants were collected and SEAP levels determined using QUANTI-Blue detection assay. Error bars represent SD. The graph shows the mean + SEM of n = 2 experiments, significance was assessed by two-way ANOVA. (d) Immune cell activation by XS15: Fresh PBMCs were cultured for 40 h in the presence of Phytohemagglutinin-L (PHA) + Pokeweed (PWM) (P + P), Pam3CysSK4 (P3CSK4), XS15 or left untreated (− ctrl.). Activated NK (left panel) and B cells (right panel) were assessed with the marker CD69 following the gating strategy: time gate, single cells (FSC-H/ FSC-A), living cells (Zombie-Aqua/ FSC-A), lymphocytes (FSC-A/ SSC-A); B-cells were defined as CD14neg CD3neg CD19+ cells and NK cells as CD14neg CD3neg CD19neg CD56+ cells. Healthy donors (n = 6), means are shown, significance was assessed by one-way ANOVA. (e) Dendritic cell (DC) stimulation by XS15: DCs were differentiated from blood monocytes and then matured as described in the material and methods section. Gating strategy was: time gate, single cells (FSC-H/ FSC-A), living cells (Zombie Aqua/ FSC-A). Upper panel: scatter plots for healthy donors (n = 6), means are shown significance was assessed by one-way ANOVA. Lower panel: modal histograms and median fluorescences for one representative donor. Medium control without maturation cocktail = − ctrl. Standard maturation cocktail = Mat. (f) Induction of cytokine release by XS15: Anticoagulated whole blood was incubated with XS15 (10 μg/ml) as well as LPS (100 ng/ml) and PHA (2 μg/ml)/ PWM (1 μg/ml) as positive (+ ctrl.) and medium only as negative controls (− ctrl.) and supernatants harvested after 20 h. Multiplexed bead-based sandwich immunoassays were performed using a LUMINEX device with a 42-analyte panel. Exemplary findings obtained in three healthy donors (HD) for IL-8 (left), MCP1 (middle) and MIP-1β (right) are shown with means. HD1 (blue square) designates the vaccinated volunteer characterized in more detail subsequently. Additional results are provided in Additional file 7: Table S1. In case of saturation, the upper limit of quantification (ULOQ) was assigned. p ≤ 0.05*; **p ≤ 0.01; ***p ≤ 0.001