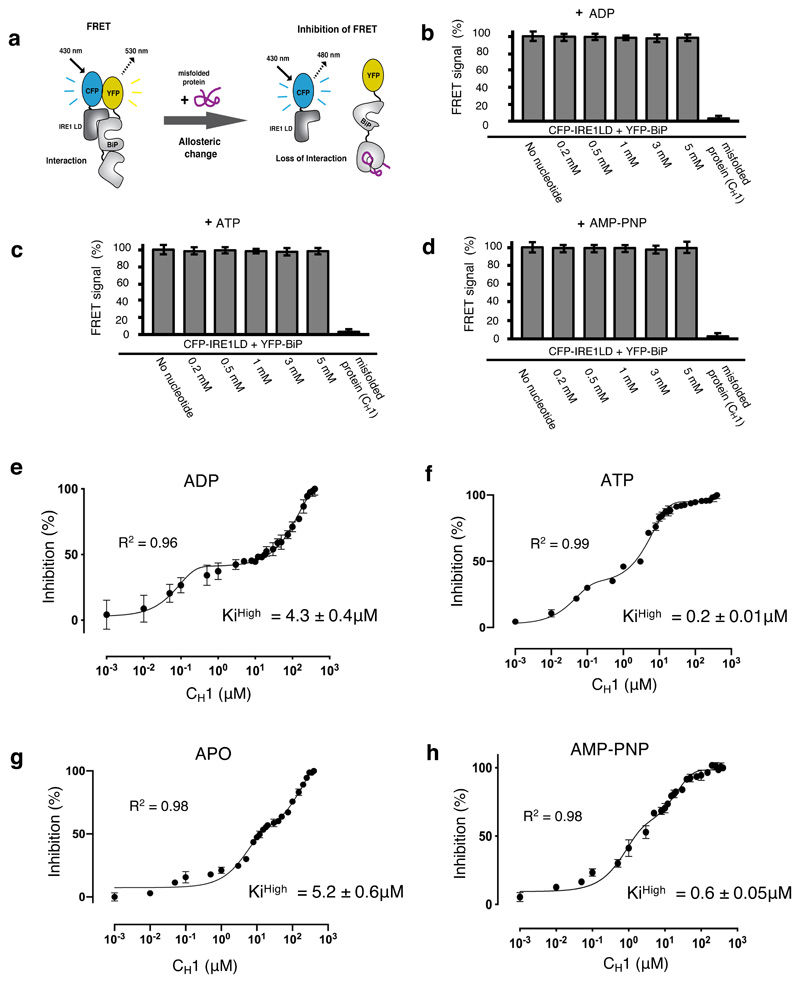
Figure 5. ATP – but not ADP – primes and facilitates the release of BiP from IRE1 LD.
a, Experimental design for three-component in vitro UPR FRET assay22,32 used to measure the inhibition of CFP-IRE1 – YFP-BiP complex by misfolded protein in the presence and absence of nucleotides. b-d, Bar graph showing FRET signal observed upon addition of ADP (b), ATP (c) or AMP-PNP (d). In each case, FRET inhibition due to dissociation of the complex was solely dependent on binding of misfolded protein CH1. Data are mean ± s.d., n=9 independent experiments. e-h, FRET signal inhibition (%) as a function of the CH1 concentration in the absence and presence of different nucleotides: ADP (e), ATP (f), APO (g) and AMP-PNP (h). Data were fitted to exponential curve (two phase association) and the IC50 (equivalent to half-life) for each phase was deduced. The IC50 measurement was then used to calculate the Ki32. The ATP bound BiP-IRE1 LD complex required 21-fold less misfolded protein to facilitate full dissociation of complex than the ADP bound state, suggesting a priming effect towards misfolded protein on ATP addition. Data are mean ± s.d., n = 9 independent experiments. Source Data are available online. The IC50 and inhibition constant values for both low and high concentration phases in the absence and presence of different nucleotides are in Supplementary Table 7.