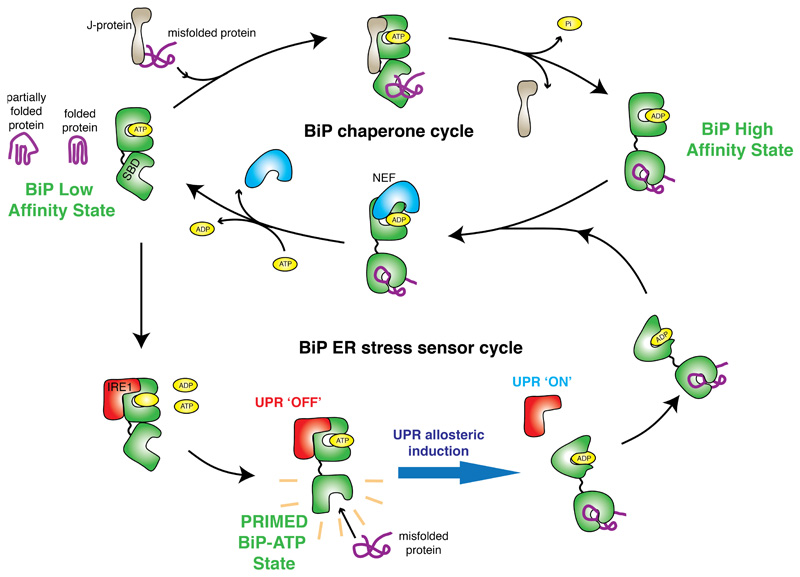
Figure 6. Mechanism of BiP function encompassing its chaperone and ER stress sensor cycles.
Interaction with UPR proteins switch BiP from chaperone cycle to ER stress sensor cycle by preventing binding to cochaperone and NEF proteins, with loss of BiP ATPase stimulation. The interaction between IRE1 LD is mediated via BiP NBD, whilst dissociation of complex is dependent on misfolded protein binding to BiP SBD. The BiP-IRE1 complex in the ATP bound state, has a greater sensitivity for misfolded protein, thereby facilitating release. By contrast the complex is desensitized to misfolded protein when bound to ADP. The release of BiP from UPR proteins via conformational change21,22 – is coupled to UPR activation34, enabling BiP to interact with cochaperones and revert to its chaperone cycle with misfolded protein attached.