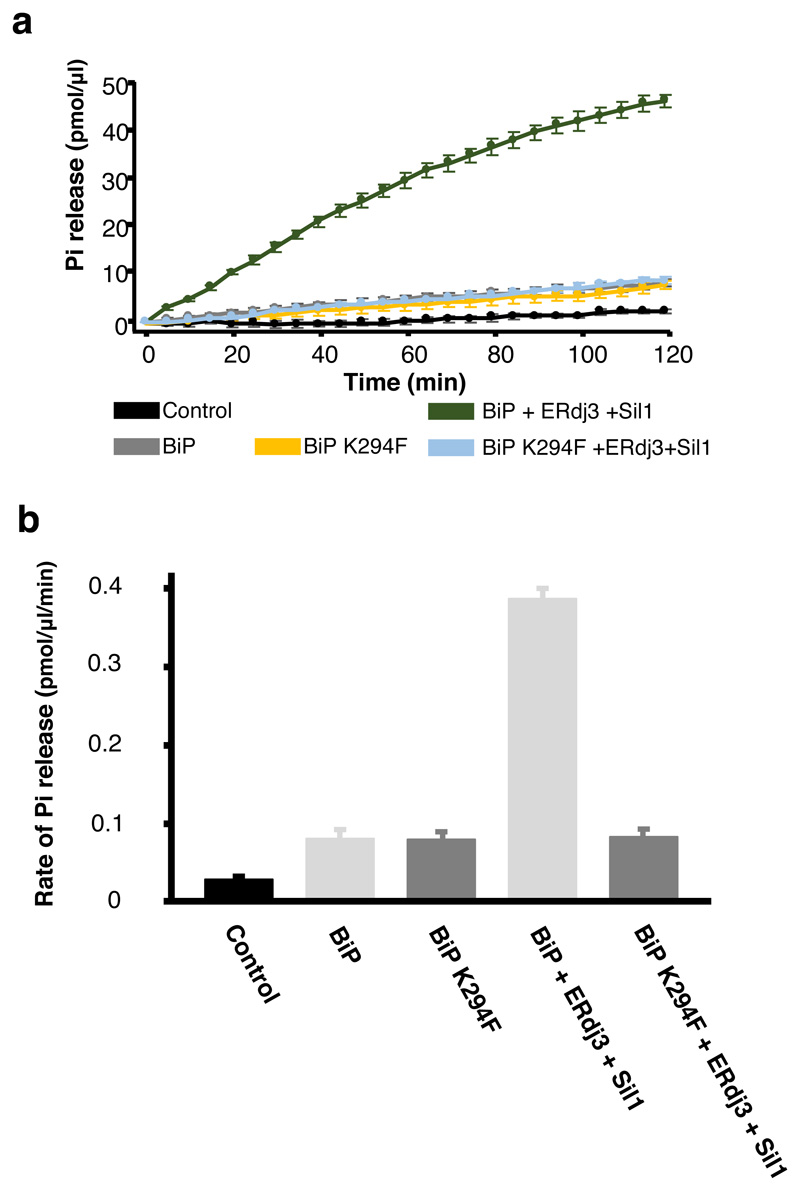
Extended Data Fig. 2. Mutant BiPK294F has same basal ATPase activity as BiPWT.
(a) BiPK294F ATPase activity on addition of co-chaperones. (b) A comparison of the rate of ATPase activity for BiP and BiPK294F. The K294F mutation based within the BiP NBD, had no effect on inherent BiP ATPase, but prevents ATPase stimulation consistent with-it inhibiting co-chaperone binding. Statistics as in Figure 1, source data available online.