Abstract
Inflammation involving migration of immune cells across the damaged blood–brain barrier (BBB), activation of resident innate microglia and production of inflammatory humoral mediators such as cytokines and chemokines play a critical role in the pathogenesis of ischemic stroke. Cell-cell signaling involved in the process also includes checkpoint interaction between programmed death receptor (PD1) and programmed death ligands, PDL1 and PDL2. Based on our previous studies showing reduced MCAO infarct volumes in PDL2 deficient mice, we evaluated the ability of anti-PDL2 mAb to treat MCAO in male and female C57BL/6 mice. We found that anti-PDL2 neutralizing antibody treatment of MCAO significantly reduced infarct volumes in male mice but had no protective effects in female mice even at a 5-fold increased dose of anti-PDL2 mAb. The protection in male mice was likely mediated by reduced percentages in the spleen of PDL2+CD19+ B cells, PDL1+CD4+ T cells and CD86+CD11b+ macrophages in concert with reduced expression of PDL1 and TNFα and continued expression of CD206, in the injured ipsilateral brain hemisphere. The lack of a therapeutic benefit of anti-PDL2 on stroke-induced infarct volumes in female mice was reflected by no detectable reduction in expressed PDL2 or PDL1 and an increased frequency of Th1 and Th17 pro-inflammatory T cell subsets in the spleen, an effect not seen in PDL2 mAb treated males. This result potentially limits the utility of anti-PDL2 mAb therapy in stroke to males but underscores the importance of meeting the STAIR requirements for development of new stroke therapies for both sexes.
Keywords: MCAO, Anti-PDL2 mAb, Checkpoint regulation, Sex differences
Background
Inflammation plays a critical role in the pathogenesis of ischemic stroke. Key features of neuroinflammation are the invasion of peripheral immune cells across the damaged blood–brain barrier (BBB), activation of resident innate immune cells, microglia, and production of inflammatory humoral mediators such as cytokines and chemokines (Iadecola and Anrather 2011). Although cells of the innate immune system, especially neutrophils and monocytes remain central, recent studies have demonstrated that T cells also contribute to tissue damage. Indeed, T cell-deficient mice develop smaller infarct volumes and improved functional outcomes vs. wild-type (WT) mice (Hurn et al. 2007; Kleinschnitz et al. 2010; Shichita et al. 2009; Yilmaz et al. 2006). However, the mechanisms of T cell-mediated brain injury following stroke remain unclear. Various immunotherapeutic strategies have been tested to lessen inflammatory responses after brain injury in order to improve stroke outcome. In addition to blocking immune cell infiltration into the ischemic, damaged brain (Becker et al. 2001; Liesz et al. 2011; Zhou et al. 2013), endogenous regulatory and immunosuppressive pathways have been intensively investigated (Iadecola and Anrather 2011; Li et al. 2013; Liesz et al. 2013). Clearly, a better understanding of regulatory approaches is warranted for translation into clinical practice.
Programmed Death-1 (PD1)(CD279), expressed on activated T, B and myeloid cells elicits inhibitory signals upon co-ligation of PD1 ligands, PDL1 (CD274) or PDL2 (CD273), during activation of the T cell receptor (TCR) and plays a key role in immune tolerance (Agata et al. 1996; Dong et al. 1999; Greenwald et al. 2005; Ishida et al. 1992; Latchman et al. 2001) by inhibiting expansion and differentiation of naive self-reactive T cells into effector T cells (Francisco et al. 2010) and inhibiting secondary immune reactions (Chen 2004; Sharpe et al. 2007). However, a number of recent studies suggest a pro-autoimmune rather than a suppressive function for PDL1. For example, transgenic over-expression of PDL1 on pancreatic beta cells enhances rather than suppresses autoimmunity (Subudhi et al. 2004). Conversely, intracerebral microinjections of PDL1−/− dendritic cells (DCs) produced the unexpected benefit of ameliorating experimental autoimmune encephalomyelitis (EAE) (Zozulya et al. 2009). A pivotal role for PD1/PDL in maintaining an immunosuppressive tumor environment has also emerged, thus implicating this pathway as a target for cancer therapy (Topalian et al. 2012). Our own studies in MCAO, however, have demonstrated inhibitory effects for PD1 (Ren et al. 2011), but enhancing effects for PDL1 and PDL2 (Bodhankar et al. 2013). These results suggest anti-PDL1/L2 mAb blockade could have therapeutic potential for treatment of human stroke.
It is now apparent that males and females respond to stroke differently. Females have a lower incidence of stroke and are more protected from ischemia vs. males (Alkayed et al. 1998; Murphy et al. 2004; Sudlow and Warlow 1997). Females also exhibit less stroke-induced splenic atrophy, which appears to correlate with reduced infarct volume (Banerjee et al. 2013). However, females suffer higher rates of disability and handicap over time compared to males (Di Carlo et al. 2003). The underlying sex-linked molecular and inflammatory mechanisms have not been well studied. In retrospect, it is not surprising that therapies such as Tirilazad that were tested exclusively in male rodents failed in human clinical trials that included both sexes, with a worse functional outcome in females (Tirilazad mesylate in acute ischemic stroke: A systematic review. Tirilazad International Steering Committee 2000). Hence, it is now a priority to test potential stroke treatments in both sexes of mice.
In order to investigate PDL1 and PDL2 interaction after stroke, young male C57BL/6 mice were treated with either phosphate buffered saline (PBS) or 200 μg anti-PDL2 mAb 4 h after 60 min transient MCAO and female mice were treated with PBS or or 1 mg anti-PDL2 mAb. Infarcts and splenic immune responses were analyzed at 96 h after reperfusion.
Methods
Animals
Male and female C57BL/6 J mice, age 8–10 weeks, were purchased from The Jackson Laboratory (Sacramento, CA). Mice were given food and water ad libitum and kept on a 12 h light/dark cycle in climate controlled housing. Animals were cared for according to institutional guidelines in the animal resource facility at the Oregon Health and Science University, Portland, OR.
Middle cerebral artery occlusion
Transient focal ischemia was induced in male and female mice for 60 min by reversible middle cerebral artery occlusion (MCAO) as previously described (Zhu et al. 2015). MCAO was induced via the intraluminal filament technique as described previously with slight modifications (Zhu et al. 2010). Mice were anesthetized with isoflurane (5% induction; 1–2% maintenance) throughout surgery and during a 60-min vascular occlusion until filament withdrawal and initiation of reperfusion. Rectal temperature was monitored and maintained at 36.5 ± 0.5 °C throughout surgery with a warm water pad and a heating lamp. Cortical blood flow (CBF) was monitored by laser-Doppler flowmetry (LDF; Model DRT4, Moor Instruments Ltd., Oxford, England). The right lateral common carotid artery (CCA) was exposed and temporarily ligated. The right external carotid artery (ECA) was ligated and cauterized. MCAO was accomplished by inserting a 6–0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with a heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip into the internal carotid artery (ICA) via the ECA stump until the tip of filament reached the origin of the middle cerebral artery (MCA). The successful occlusion of MCA was confirmed by a drop, followed by sustained CBF of less than 30% of the baseline. The filament was withdrawn and the CCA was released to allow for reperfusion at 60-min occlusion. The mice were then allowed to recover from anesthesia and survived for 96 h following the onset of ischemia. Animals were excluded if CBF failed to drop below 30% of the baseline during MCAO or due to sub-arachnoid hemorrhage.
Treatment with anti-PDL2 neutralizing antibody
Male mice were treated with either 200 μl sterile PBS or 200 μg of anti-PDL2 antibody (TY25, BioXCell, Lebanon, NH) in 200 μl of PBS intraperitoneally (i.p.) 4 h after occlusion. Female mice were treated with either 300 μl PBS or 1 mg of anti-PDL2 antibody in 300 μl of PBS i.p. 4 h after occlusion.
Infarct volume quantification
Infarct volume measurement was performed as previously described (Zhu et al. 2015). Animals were euthanized and brains were removed 96 h after MCAO. Infarct volumes were measured after staining four slices of 2-mm thick coronal sections with a 1.2% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO) and incubated for 15 min at 37 °C, and then fixed in 10% buffered formalin overnight. Both sides of each stained slice were photographed and evaluated by SigmaScan Pro 5.0 (Jandel, San G, Rarael, CA, USA). Infarct volume was expressed as a percentage of contralateral structure (cortex, striatum, and hemisphere). To account for the effect of edema, infarct volume was calculated by subtracting the ipsilateral non-infarct region from the total contralateral structure volume, and dividing the difference by the contralateral volume (Zhang et al. 2013).
Isolation of immune cells from the spleen
Spleens were processed into single cell suspensions by passing the spleen through a 100 μm nylon mesh (BD Falcon, Bedford, MA) into RPMI 1640. Red blood cells were lysed with 1× red cell lysis buffer (eBioscience, Inc., San Diego, CA). After being washed with RPMI 1640 the cells were counted on a Cellometer Auto T4 cell counter (Nexcelom, Lawrence, MA). Cells were centrifuged and resuspended in staining buffer (PBS with 0.1% NaN3 and 1% bovine serum albumin) for staining.
Flow cytometry
For flow cytometric analysis, cells were placed in staining buffer at a concentration of 1 × 106 cells/ml for splenocytes and 2 × 105 cell/ml for brain and blood cells. Cells were blocked with rat anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Bioscience, San Jose, CA) and then incubated, protected from light, with various combinations of fluorescently tagged antibodies. To assess cell survival, 7-aminoactinomycin D (7AAD) viability dye was used in some samples. For intracellular and FoxP3 staining, the samples were fixed with 4% paraformaldehyde and washed. FoxP3 staining was done using the fixation/permeabilization reagents per the manufacturer’s instructions (eBioscience). For intracellular staining, cells were resuspended in 1X permeabilization buffer (BD Bioscience) and incubated with antibodies or isotype controls. The samples were then run on a BD Accuri™ C6 (BD Bioscience) under a four color (FITC, PE, PerCP/PECy5.5, and APC) fluorescence flow cytometry analysis.
The following antibodies were used: CD4 (GK1.5), PDL1 (M1H5), CD11b (M1/70), CD19 (1D3), CD1d (1B1), CD23 (B3B4), TIM1 (RMT1–4), CD138 (281–2), CD25 (PC61), CD86 (GL1), CD21 (7G6), IA/IE (2G9), TNFα (18139A), NOS2 (CXNFT), CD206 (CO68C2) (BD Biosciences), CD44 (1 M7), FoxP3 (FJK-16 s), IRF4 (3E4), IFNγ (XMG1.2) (eBiosciece), CD9 (MZ3), CD5 (53–7.3) (Biolegend), and PEARG1 (R & D Systems, Minneapolis, MN).
RT-PCR
Total RNA from sorted brains was isolated using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript II Reverse Transcriptase cDNA synthesis kit (Life Technologies, Grand Island, NY). Quantitative real time PCR was performed using the Stepone Plus Real-Time PCR System with TaqMan Gene Expression Array Plates for mouse immune response. cDNA from three mice per group was quantified for gene array plates. Individual samples were analyzed in triplicate for Arg-1, CD206, TNFα and NOS2 (Life Technologies, Grand Island, NY). The GAPDH housekeeping gene was used as an endogenous control. Results were analyzed using Expression Suite Software (Life Technologies, Grand Island, NY).
Statistics
Data were analyzed using Prism software (GraphPad Software, La Jolla, CA) using ANOVA with a Fischer’s Least Significant Difference post-hoc test or Student’s t test when appropriate. A p value of ≤0.05 was considered significant. Data are represented as mean ± standard error of the mean (SEM). All analyses were carried out in blinded fashion.
Results
Anti-PDL2 treatment reduces infarct volumes in male but not female mice following 60 min MCAO
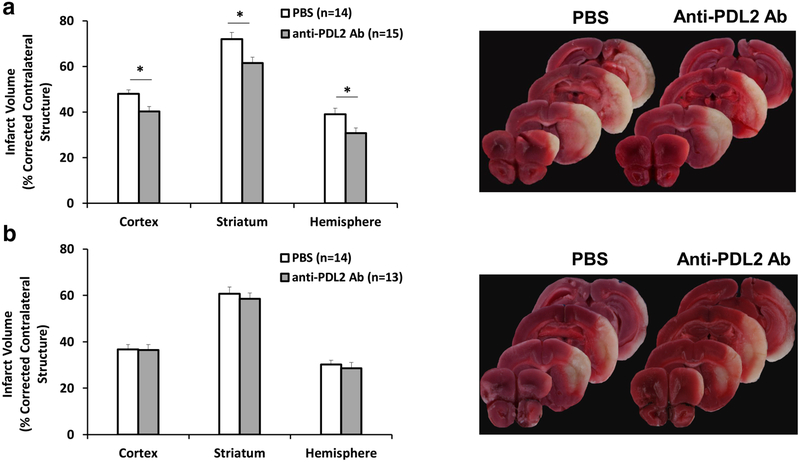
Treatment of male mice with 200 μg anti-PDL2 neutralizing mAb 4 h after 60 min of transient MCAO significantly reduced the infarct volumes at 96 h compared to vehicle treated male mice (Fig. 1a). In contrast, female mice treated with an increased 1 mg dose of anti-PDL2 mAb after MCAO had no difference in infarct volumes at 96 h (Fig. 1b).
Fig. 1. Anti-PDL2 treatment protects male but not female mice following 60 min MCAO.
Infarct volume was assessed in male and female mice 96 h after 60 min of MCAO in mice treated with vehicle or an anti-PDL2 neutralizing antibody 4 h after occlusion. Male mice (a) had significantly lower infarct volumes within the cortex, striatum, and the entire hemisphere when treated with 200 μg of anti-PDL2 neutralizing antibody (n = 15) compared to vehicle treated mice (n = 14). Female mice did not have significantly different infarct volumes between the vehicle (n = 14) and 1 mg of anti-PDL2 neutralizing antibody (n = 13) treatment groups in the cortex, striatum, or total hemisphere (b). *p < 0.05
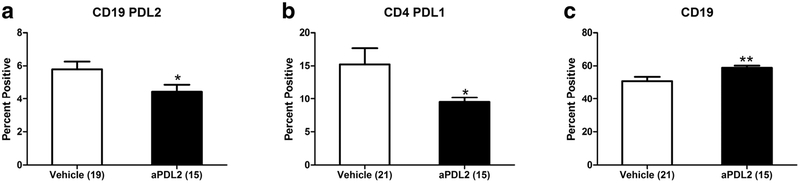
Anti-PDL2 antibody treatment after MCAO significantly affected levels of male but not female mouse splenic CD19+PDL2+ B cells as well as total CD19+ B cells and PDL1+CD4+ T cells
Treatment of male mice with anti-PDL2 mAb after MCAO significantly decreased the expression of PDL2+CD19+ B cells (Fig. 2a) as well as PDL1+CD4+ T cells (Fig. 2b) compared to vehicle treated males. The anti-PDL2 treatment also significant increased the levels of total CD19+ splenic B cells (Fig. 2c). In contrast, there were no significant changes in PDL1, PDL2 or B cell expression in spleens from PDL2-treated female mice (data not shown).
Fig. 2. Anti-PDL2 antibody treatment changes the male splenocyte subtypes, PDL1 and PDL2 expression but not in female mice.
Treatment with an anti-PDL2 neutralizing antibody (n = 15) significantly decreased the frequency of PDL2 on CD19+ splenocytes compared to vehicle treated mice (n = 19) 96 h post-MCAO (a). There was also a significant decrease in the frequency of PDL1 on CD4+ splenocytes in the anti-PDL2 neutralizing antibody group (n = 15) compared to vehicle treated mice (b; n = 21). In the spleens of male mice treated with anti-PDL2 neutralizing antibody after MCAO (n = 15) there was a significant increase in the frequency of CD19+ cells compared to vehicle treated mice (c; n = 21). *p < 0.05; **p < 0.01
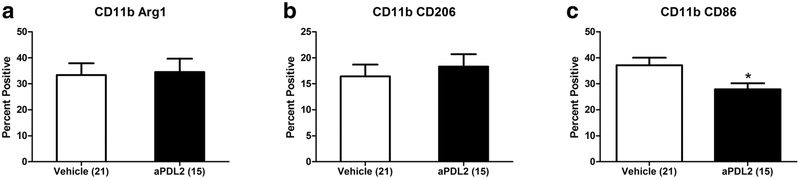
Anti-PDL2 antibody treatment after MCAO decreased activated splenic macrophages in male but not in female mice
Spleen cells from male mice treated with anti-PDL2 after MCAO did not show any differences in in expression of the anti-inflammatory macrophage makers, Arg-1 (Fig. 3a) and CD206 (Fig. 3b) compared to vehicle treated males. However, the frequency of splenic macrophages expressing the proinflammatory CD86 marker was significantly decreased after anti-PDL2 antibody treatment compared to vehicle treated males (Fig. 3c). In contrast, there were no significant changes in any of these activation markers on splenic macrophages from female mice (data not shown).
Fig. 3. Anti-PDL2 decreases activated splenic macrophages in male mice but not in female mice.
Male mice treated with anti-PDL2 neutralizing antibody 4 h post-MCAO did not have any significant differences in the frequency of anti-inflammatory splenic macrophages as indicated by the frequency of CD11b+ Arg-1+ cells (a) or CD11b+ CD206+ cell (b). There was a significant decrease in the frequency of pro-inflammatory CD11b+ CD86+ cells in the spleens of anti-PDL2 neutralizing antibody treated mice (n = 15) compared to vehicle treated mice (n = 21) 96 h post-MCAO (c). *p < 0.05
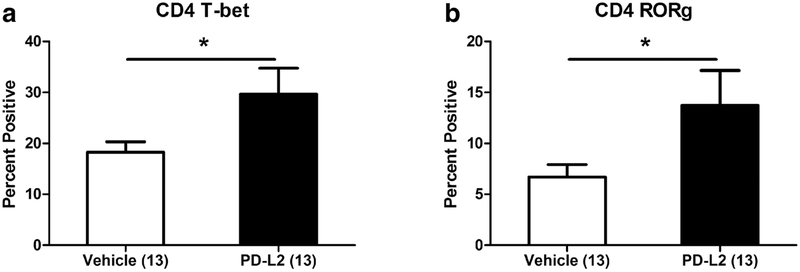
Treatment with anti-PDL2 antibody after MCAO increases splenic Th1 and Th17 cells only in female mice
There were significant increases in the frequencies of splenic CD4+T-bet+ (Th1) cells (Fig. 4a) and CD4+RORγ+ (Th17) cells (Fig. 4b) in the anti-PDL2 antibody treated female mice compared to vehicle treated mice 96 h post-MCAO. Conversely, there were no changes in the frequencies of CD4+T-bet+ Th1 cells or CD4+RORγ+ Th17 cells in the spleens of male mice treated with vehicle or anti-PDL2 antibody (data not shown).
Fig. 4. Treatment with anti-PDL2 antibody after MCAO increases splenic Th1 and Th17 cells only in female mice.
In the spleens of female mice treated with anti-PDL2 neutralizing antibody after MCAO (n = 13) there was a significant increase in the frequency of CD4+T-bet+ (Th1) cells compared to vehicle treated female mice (a; n = 13). There was an increase in the frequency of CD4+RORγ+ (Th17) cells compared to the vehicle treated females (b). *p < 0.05
PDL1, PDL2 and PD1 expression in the brains of male mice after MCAO
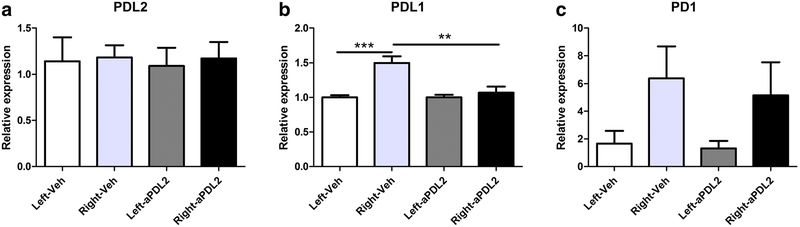
Although no changes in expression of PDL2 were observed in ipsilateral or contralateral hemispheres in either the vehicle treated or anti-PDL2 treated male mice after MCAO (Fig. 5a), there was a significant increase in PDL1 expression in the ipsilateral vs. contralateral hemisphere of vehicle treated mice that was notably absent in the ipsilateral hemisphere after anti-PDL2 treatment (Fig. 5b). The expression levels of PD1 were not different between hemispheres in either treatment group (Fig. 5c).
Fig. 5. PD1, PDL1 and PDL2 expression in the brains of male stroked mice.
In the brains of male mice 96 h post-MCAO, no changes in expression of PDL2 were observed in ipsilateral (right) or contralateral (left) hemispheres in either the vehicle treated (n = 6) or anti-PDL2 treated (n = 6) male mice after MCAO (a) There was a significant increase in PDL1 expression in the ipsilateral hemisphere of vehicle treated mice compared to the contralateral hemisphere. PDL1 expression in the anti-PDL2 treated ipsilateral hemisphere was also significantly decreased compared to the vehicle treated ipsilateral hemisphere of the mice (b). There were not any significant differences in the expression of PD1 (c) in either hemisphere or between both treatments. **p < 0.01; ***p < 0.001
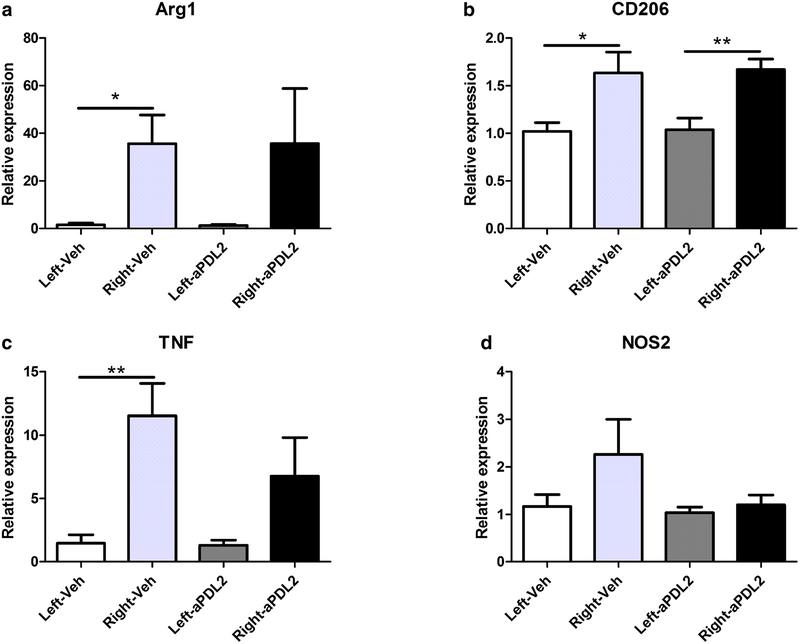
Treatment with anti-PDL2 antibody after stroke decreases TNFα expression in the brains of male mice
Real time PCR was used to evaluate gene regulation in male brain hemispheres in anti-PDL2 antibody treated compared to vehicle treated groups 96 h after MCAO. Anti-inflammatory genes, Arg1 (Fig. 6a) and CD206 (Fig. 6b), were significantly increased in the ipsilateral (right) hemispheres compared to the contralateral (left) hemispheres of the vehicle groups, but only CD206 but not Arg1 remained significantly increased in the ipsilateral hemisphere of the anti-PDL2 treated group (Fig. 6b). The proinflammatory marker, TNFα, was significantly increased in the ipsilateral hemisphere compared to the contralateral hemisphere in vehicle treated mice but in the anti-PDL2 treated males, TNFα expression in the ipsilateral hemisphere did not differ significantly from the contralateral hemisphere and also was nominally reduced by nearly half compared to the ipsilateral hemisphere of vehicle treated males (Fig. 6c). There were no significant differences in males in the expression of NOS2 in the vehicle treated or anti-PDL2 treated ipsilateral versus contralateral mouse brain hemispheres (Fig. 6d).
Fig. 6. Treatment with anti-PDL2 antibody after stroke decreases TNFα expression in the brains of male mice.
Microglia/macrophage activation states in the brain hemispheres were assessed using RT-PCR in male mice 96 h after MCAO. The expression of the anti-inflammatory maker Arg-1 was significantly increased in the ipsilateral (right) hemisphere compared to the contralateral hemisphere (left) in the vehicle treated mice (n = 6) but there were no differences in the Arg-1 expression between hemispheres in the anti-PDL2 neutralizing antibody (n = 6) treated group (a). The expression of CD206 was significantly increased in the ipsilateral hemispheres of the vehicle and the anti-PDL2 treated mice compared to the respective contralateral hemispheres (b). The pro-inflammatory marker TNF was significantly increased in the ipsilateral hemisphere of the vehicle treated mice compared to the contralateral hemisphere and there was not a significant difference in the expression of TNF between hemispheres in the anti-PDL2 neutralizing antibody treated mice (c). There were not any significant differences in the pro-inflammatory marker NOS2 in either the vehicle or anti-PDL2 neutralizing antibody treated mice (d). *p < 0.05; **p < 0.01
Discussion
The data presented in this study demonstrate that anti-PDL2 neutralizing antibody treatment of experimental stroke significantly reduced infarct volumes in male mice but had no protective effects in female mice even at a 5-fold increased dose of anti-PDL2 mAb. The protection in male mice was likely mediated in part by reduced percentages in the spleen of PDL2+CD19+ B cells, PDL1+CD4+ T cells and CD86+CD11b+ macrophages in concert with reduced expression of PDL1 and TNFα and continued expression of the anti-inflammatory factor, CD206, in the injured ipsilateral right brain hemisphere. The lack of a therapeutic benefit of anti-PDL2 on stroke-induced infarct volumes in female mice was reflected by no detectable reduction in expressed PDL2 or PDL1 and an increased frequency of Th1 and Th17 pro-inflammatory T cell subsets in the spleen, an effect not seen in PDL2 mAb treated males.
Our previous studies determined that MCAO lesion volumes were decreased in the ipsilateral hemisphere in PDL2 deficient mice (Bodhankar et al. 2013), thus implicating PDL2 as a potential pathogenic factor. To mimic PDL2 depletion, we treated male mice after MCAO with anti-PDL2 neutralizing mAbs and observed reduced infarct volumes in the affected brain cortex, striatum and hemisphere (Fig. 1a). In females, however, treatment with a 5-fold increase in anti-PDL2 mAb was ineffective even though the MCAO lesion volumes in vehicle treated mice appeared to be ~25% smaller than in males (Fig. 1b), thus ruling out higher disease activity as a possible cause of the inability to treat.
Cellular components were evaluated in the spleen of males and females for changes that might reflect the sex differences in treatment. In males, our studies revealed a significant reduction in PDL2+ B cells, PDL1+ T helper cells and CD86+ macrophages, but an overall increase in spleen cell numbers (Figs. 2 and 3). This potentially could be due to a reduced number of disease promoting proinflammatory CD86+ macrophages, PDL2+ B cells and PDL1+ T cells and overcompensation by regulatory B and T cell subsets that we have shown previously can ameliorate MCAO severity in the absence of PD1/PDL interactions (Bodhankar et al. 2013). In females, anti-PDL1 treatment did not affect these spleen cell subtypes, but rather promoted increased numbers of proinflammatory T-bet+ Th1 and RORγ+ Th17 effector cells (Fig. 4) that conceivably might neutralize the protective anti-PDL2 mAb effects observed in males.
In the ischemic brain hemisphere, anti-PDL2 mAb treatment did not alter PDL2 or PD1 expression, but resulted in reduced PDL1 expression significantly below that detected in the ipsilateral hemisphere from vehicle treated males (Fig. 5). Moreover, anti-PDL2 treatment did not reduce normal expression levels of CD206, although Arg1 expression may have been impacted in some mice (Fig. 6a). This suggested that these modulatory factors may have contributed to an anti-inflammatory milieu in the brain that contributed to the marked reduction in TNFα expression in the injured hemisphere (Fig. 6c).
In summary, treatment of MCAO with anti-PDL2 mAb resulted in a significant treatment effect in male mice that was not observed in female mice. This result potentially limits the utility of anti-PDL2 mAb therapy in stroke to males but underscores the importance of meeting the STAIR requirements for development of new stroke therapies for both sexes.
Acknowledgements
This work was funded by the American Heart Association grant 17GRNT33220001 (HO) and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Senior Research Career Scientist Award 1IK6BX004209 (AAV). The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The authors would like to acknowledge the following individuals for their contributions towards the completion of this manuscript: Dr. Gil Benedek, Gail Kent, Grant Gerstner, and Ha Nguyen.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8:765–772 [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD (1998) Gender-linked brain injury in experimental stroke. Stroke 29: 159–165 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H (2013) Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res 4:554–563. 10.1007/s12975-013-0268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R (2001) Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 32:206–211 [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H (2013) PD L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation 10:111 10.1186/1742-2094-10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L (2004) Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 4:336–347. 10.1038/nri1349 [DOI] [PubMed] [Google Scholar]
- Di Carlo A et al. (2003) Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 34: 1114–1119. 10.1161/01.STR.0000068410.07397.D7 [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L (1999) B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242. 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548. 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H (2007) T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab 27:1798–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796–808. 10.1038/nm.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G (2010) Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115:3835–3842. 10.1182/blood-2009-10-249078 [DOI] [PubMed] [Google Scholar]
- Latchman Y et al. (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- Li P et al. (2013) Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol 74:458–471. 10.1002/ana.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A et al. (2011) FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS One 6:e21312 10.1371/journal.pone.0021312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A et al. (2013) Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci 33:17350–17362. 10.1523/JNEUROSCI.4901-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM (2004) Stroke in the female: role of biological sex and estrogen. ILAR J 45:147–159 [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H (2011) Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 42:2578–2583. 10.1161/STROKEAHA.111.613182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8:239–245. 10.1038/ni1443 [DOI] [PubMed] [Google Scholar]
- Shichita T et al. (2009) Pivotal role of cerebral interleukin-17-producing gammadeltaTcells in the delayed phase of ischemic brain injury. Nat Med 15:946–950. 10.1038/nm.1999 [DOI] [PubMed] [Google Scholar]
- Subudhi SK et al. (2004) Local expression of B7–H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest 113: 694–700. 10.1172/JCI19210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP (1997) Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration Stroke 28: 491–499 [DOI] [PubMed] [Google Scholar]
- Tirilazad mesylate in acute ischemic stroke (2000) A systematic review. Tirilazad International Steering Committee. Stroke 31:2257–2265 [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7–H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212. 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN (2006) Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113:2105–2112 [DOI] [PubMed] [Google Scholar]
- Zhang W, Davis CM, Edin ML, Lee CR, Zeldin DC, Alkayed NJ (2013) Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS One 8:e61244 10.1371/journal.pone.0061244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W et al. (2013) Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol 23:34–44. 10.1111/j.1750-3639.2012.00614.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W et al. (2010) Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience 169:758–769. 10.1016/j.neuroscience.2010.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Dotson AL, Libal NL, Lapato AS, Bodhankar S, Offner H, Alkayed NJ (2015) Recombinant T-cell receptor ligand RTL1000 limits inflammation and decreases infarct size after experimental ischemic stroke in middle-aged mice. Neuroscience 288:112–119. 10.1016/j.neuroscience.2014.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Ortler S, Fabry Z, Sandor M, Wiendl H (2009) The level of B7 homologue 1 expression on brain DC is decisive for CD8 Treg cell recruitment into the CNS during EAE. Eur J Immunol 39:1536–1543. 10.1002/eji.200839165 [DOI] [PMC free article] [PubMed] [Google Scholar]