Abstract
Objective:
To assess right – left differences in ultrasonographic markers of ovarian morphology and determine the impact on the diagnosis of polycystic ovarian morphology (PCOM).
Design:
A cross-sectional study of data collected from 2006 to 2018.
Setting:
Four academic clinical research centers in North America.
Patients:
Women with polycystic ovary syndrome (PCOS; n = 87) and controls (n = 67).
Intervention(s):
None.
Main Outcomes Measure(s):
Follicle number per ovary (FNPO), follicle number per cross-section (FNPS), and ovarian volume (OV) were assessed in both ovaries using transvaginal ultrasonography. PCOM was identified based on recent international consensus guidelines or proposed diagnostic thresholds.
Results:
Overall, mean right – left differences were 2 follicles for FNPO (p = 0.07), 1 follicle for FNPS (p = 0.01), and 2 ml for OV (p < 0.01). FNPO showed the strongest correlation between ovaries. Its assessment in a single ovary did not impact the diagnosis of PCOM in women with PCOS. However, there were differences in the probability of uni- versus bilateral PCOM based on FNPS (p < 0.01) and OV (p < 0.01) in both groups.
Conclusions:
FNPO is the most reliable unilateral marker of PCOM in light of right – left differences in ovarian morphology. Use of FNPS or OV to define PCOM is discouraged when only one ovary is visualized.
Keywords: polycystic ovary syndrome, ultrasonography, diagnosis, intra-individual biological variation
Capsule:
Right – left differences in ovarian morphology can impact the ultrasound diagnosis of polycystic ovary syndrome. Use of follicle number per ovary, even if in a single ovary, is recommended.
INTRODUCTION
A reliable definition of polycystic ovarian morphology (PCOM) is required to identify clinical variants of polycystic ovary syndrome (PCOS) (1–3). Hallmarks of PCOM include follicular excess and increased ovarian size (4–6), which can be observed non-invasively using transvaginal ultrasonography (7). The development of higher frequency ultrasound transducers (i.e., ≥ 8 MHz) has improved the accuracy of morphologic assessments and ushered international efforts to re-evaluate the diagnostic markers and thresholds for PCOM (3,7).
Follicle number per ovary (FNPO) is now recognized as the most reliable marker of PCOM (3,7–14). The original definition of “follicular excess” (i.e., FNPO ≥ 12) (15,16) is considered obsolete and experts agree that thresholds to distinguish normal from polycystic ovaries will require revision as advances in ultrasound technology are introduced (3,7). Modern diagnostic thresholds for FNPO have been proposed by the Androgen Excess and PCOS Society for its 2014 Task Force Report on the Definition and Significance of PCOM (7) and International PCOS Network for its 2018 International Guideline on the Assessment and Management of PCOS (3). Both groups concluded that the threshold be raised to ≥ 25 (7) and ≥ 20 follicles per ovary (3), respectively. The different values reflect the use of different upper limits for FNPO in a control population – albeit the best approach for establishing a diagnostic threshold is debatable (7). Nevertheless, the acceptance of higher thresholds for FNPO is critical to reliably evaluate PCOS with newer imaging technology.
Other markers of PCOM (i.e., follicle number per cross-section (FNPS) and ovarian volume (OV)) provide inferior diagnostic potential for PCOS (8–14). However, FNPS and OV could serve as suitable alternatives to FNPO when the transabdominal route is preferred or image quality is reduced (3,7,14). Notably, FNPS has never been included in international definitions of PCOM (3,7,16), but remains relevant to study given its widespread use as a surrogate marker of follicular excess in clinical practice (7). Further, the definition of “increased ovarian size” (i.e., OV ≥ 10 ml) has not changed since the Rotterdam consensus (3,7,16), suggesting that OV is robust against advances in ultrasound technology.
Despite efforts to refine diagnostic markers and thresholds for PCOM (3,7,16), there are limited data on the appropriateness of technical standards for conducting ultrasound assessments. Historically, diagnostic test studies have derived thresholds from mean measurements of FNPO, FNPS, and OV, with values averaged between the right and left ovaries (8–13,15). Consensus statements have extended the findings to indicate that unilateral elevation in any ultrasonographic marker is sufficient to meet the definition of PCOM (3,7,16). The recommendation is presumably based on early reports that bilateral polycystic ovaries were the unifying feature of women with PCOS (4,17). However, application of mean thresholds to one side relies on the assumption that there are no measurable, biological differences between the right and left ovaries. Few studies have considered right – left differences in ovarian morphology in women of reproductive age. It stands to reason that lateralization of follicular excess or ovarian size would compromise the diagnostic potential of PCOM for PCOS, particularly if only one ovary is scanned. Therefore, the present study aimed to assess right – left differences in ultrasonographic markers of ovarian morphology (i.e., FNPO, FNPS, and OV) and determine the impact on the diagnosis of PCOM.
MATERIALS AND METHODS
Ethical Considerations
The present study involved secondary analysis of pooled data from seven separate protocols. Studies were conducted at four clinical research centers in North America from 2006 to 2018. Protocols were approved by the Biomedical Research Ethics Review Board at the University of Saskatchewan (SK, Canada) and Institutional Review Boards at Cornell University, University of Rochester, and Weill Cornell Medicine (NY, USA). Informed consent was obtained before study procedures were initiated. Some participants have been evaluated in previous publications (13,14,18–21), including one study that proposed ultrasonographic thresholds for PCOM (13).
Study Participants
Women of reproductive age (18 – 38 y) were recruited from the general population. Women who were pregnant, lactating, or taking hormonal contraceptives or insulin-sensitizers were not eligible to participate in any of the protocols. Those with thyroid abnormalities, hyperprolactinemia, history of oophorectomy, or limited visualization of the ovaries on ultrasound were excluded from the present study. Women with PCOS (n = 87) were identified by the combined presence of oligomenorrhea (3) and hyperandrogenism (22). Hyperandrogenism was defined as a modified hirsutism score ≥ 7 (23) or serum total testosterone concentration ≥ 61.5 or ≥ 127.1 ng/dl (depending on the protocol and hormone assay used). Thresholds reflected the 95th percentiles of modified hirsutism score and serum total testosterone concentration in a reference cohort. Women with regular cycles and no evidence of hyperandrogenism were included as controls (n = 67).
Ultrasonographic Assessments
Participants were evaluated with high-resolution transvaginal ultrasonography. Ultrasonographic data were obtained using UltraSonix RP (Version 2.3.5; UltraSonix Medical Corporation, Vancouver, BC, Canada) and GE Voluson ultrasound machines (E8, S6, or S10 Series; GE Healthcare, Milwaukee, WI, USA) with 5 – 9 or 6 – 12 MHz multifrequency transducers. Ultrasound examinations were conducted in the early follicular phase of the menstrual cycle (controls) or when no dominant follicles or active corpora lutea were present (PCOS). Whole ovaries were scanned from their inner to outer margins in the longitudinal plane. Two-dimensional cineloops were archived for offline analysis using customized imaging software (Sante DICOM Editor, Santesoft LTD, Athens, Greece). Images were reviewed by one of three investigators. Each investigator demonstrated strong inter-rater agreement in FNPO as part of an internal reliability assessment.
Endpoints of interest included FNPO, FNPS, and OV. FNPO was assessed throughout each ovary by imposing a programmable grid onto the viewing window and making focused follicle counts in each grid section (24). FNPS and OV were obtained in the largest cross-section of each ovary (14). OV was calculated using the simplified formula for a prolate ellipsoid (16). If a cystic structure was detected (e.g., hemorrhagic anovulatory follicle, corpus luteum, or unspecified cyst), then OV was excluded for both ovaries (n = 5). FNPO, FNPS, and OV were tabulated for each ovary. Mean values between ovaries were calculated and rounded to the nearest whole numbers. Sided and mean values were ascribed a morphologic diagnosis based on recent international consensus guidelines or proposed thresholds for PCOM. PCOM was defined by an FNPO ≥ 20 (3), FNPO ≥ 25 (7), FNPS ≥ 9 (13), or OV ≥ 10 ml (3,7,16).
Biochemical Assays
Total testosterone was measured with two different liquid chromatography tandem-mass spectrometry (LC/MS/MS)-based methods. LC/MS/MS was used in combination with isotope dilution for samples collected at the University of Saskatchewan (n = 59) (18) and with solid phase extraction for samples collected at Cornell University, University of Rochester, and Weill Cornell Medicine (n = 95) (21). Intra- and inter-assay coefficients of variation were <7.5% for both methods. However, the pooled data showed a bimodal distribution, wherein total testosterone concentrations were significantly higher in samples treated with isotope-dilution versus solid phase extraction (Mann-Whitney U test; p < 0.01). Although LC/MS/MS is recommended for assessments of total testosterone in women, there can still be considerable variability between methods and lack of standardization between laboratories (25). To avoid misrepresentation of biochemical data, participants were categorized as having “normo-” or “hyperandrogenemia” based on assay-specific thresholds (described in Study Participants). Only the proportion of women with biochemical hyperandrogenism is reported herein.
Statistical Analyses
Statistical analyses were performed with JMP Pro 12 (SAS Institute Inc., Cary, NC). The threshold for statistical significance was set at p < 0.05. All endpoints were non-normally distributed. Demographic and diagnostic characteristics were compared between groups using Mann-Whitney U or Fisher’s Exact tests. Right – left differences in FNPO, FNPS, and OV was assessed using Wilcoxon Signed Rank tests. Correlations were considered with Spearman’s rank coefficients. Differences in the probability of uni- versus bilateral PCOM were determined using McNemar’s Test. Sensitivity analyses were also undertaken in the entire cohort to confirm that findings were not impacted by methodologic or physiologic factors. FNPO, FNPS, and OV were compared across sites, ultrasound machines, and maximum transducer frequencies (i.e., 9 vs. 12 MHz) using the Steel-Dwass test. The absence of differences between sites and technologies provided justification for pooling data across protocols (All: p > 0.10). Mean right – left differences in FNPO, FNPS, and OV were compared between women according to obesity, gravidity, and parity status using Wilcoxon Rank Sum tests. The absence of differences between binary categories suggested that study outcomes were not impacted by the tested physiologic factors (All: p > 0.05). Post hoc calculations revealed that the present study had 99.9% power to detect the observed intra-individual differences in FNPO, FNPS, and OV (α = 0.05).
RESULTS
Study Participant Characteristics
Demographic and diagnostic characteristics are reported in Table 1. Compared to controls, women with PCOS were of similar ages (p = 0.61), but exhibited higher body mass indices, longer menstrual cycles, and higher modified hirsutism scores (All: p < 0.01). The proportion of women with hyperandrogenemia was greater in the PCOS group (38%) versus control group (0%) (p < 0.01). Mean FNPO, FNPS, and OV were higher in women with PCOS (All: p < 0.01) (Table 1).
Table 1.
Demographic and diagnostic characteristics of the study participants
| PCOS (n = 87) | Control (n = 67) | |
|---|---|---|
| Age (y) | 27 (23–30) | 27 (23–31) |
| Body mass index (kg/m2) | 32.0 (23.7–38.2) | 23.6 (21.5–27.2)* |
| Menstrual cycle length (d) | 75 (47–146) | 29 (28–32)* |
| Modified hirsutism score | 11 (8–14) | 3 (1–5)* |
| Proportion with biochemical hyperandrogenism | 33 / 87 (38%) | 0 / 67 (0%)* |
| Mean FNPO | 45 (34–60) | 25 (16–32)* |
| Mean FNPS | 10 (8–13) | 7 (5–9)* |
| Mean OV (ml) | 11 (9–15) | 7 (6–9)* |
Data are presented as median (interquartile range) or n / total n (percent). Data related to ultrasonographic features reflect the mean of recorded values from the right and left ovaries rounded to the nearest whole number.
Within rows, an asterisk denotes a significant difference between groups based on the Mann-Whitney U (for continuous variables) or Fisher’s Exact test (for categorical variables), p < 0.05.
Abbreviations: FNPO, follicle number per ovary; FNPS, follicle number per cross-section; OV, ovarian volume.
Intra-Individual Variation in FNPO, FNPS, and OV
In the entire cohort (n = 154), mean differences between ovaries (i.e., within individuals) were 2 follicles for FNPO (95% CI: 0 – 5; p = 0.07), 1 follicle for FNPS (95% CI: 0 – 1; p = 0.01), and 2 ml for OV (95% CI: 2 – 3; p < 0.01). On average, the right ovary contained 5% more follicles and measured 20% larger than the left ovary.
Right – left differences in FNPO, FNPS, and OV are presented for each group in Table 2. Mean differences between ovaries were 5 follicles for FNPO (p = 0.02), 1 follicle for FNPS (p < 0.01), and 3 ml for OV (p < 0.01) in women with PCOS, and 0 follicles for FNPO (p = 0.77), 0 follicles for FNPS (p = 0.60), and 2 ml for OV (p < 0.01) in controls. Significant correlations were detected between ovaries – both across markers and within groups. Of the three markers, FNPO showed the strongest correlation between ovaries (PCOS: ρ = 0.73, p < 0.01; Controls: ρ = 0.68, p < 0.01). FNPS and OV had moderate correlations between ovaries in women with PCOS (FNPS: ρ = 0.57, p < 0.01; OV: ρ = 0.68, p < 0.01), but showed relatively weak relationships in controls (FNPS: ρ = 0.48, p < 0.01; OV: ρ = 0.31, p = 0.01) (Table 2).
Table 2.
Right – left differences in ultrasonographic features in controls and women with PCOS
| Right Ovary | Left Ovary | MD ± SE (95% CI) | Correlation | |
|---|---|---|---|---|
| PCOS (n = 87) | ||||
| FNPO | 45 (33–69) | 43 (32–54) | 5 ± 2 (1, 8)* | 0.73* |
| FNPS | 11 (8–13) | 10 (7–12) | 1 ± 0 (0, 2)* | 0.57* |
| OV | 13 (9–17) | 10 (7–14) | 3 ± 1 (2, 4)* | 0.59* |
| Control (n = 67) | ||||
| FNPO | 23 (16–32) | 24 (15–33) | 0 ± 1 (−2, 2) | 0.68* |
| FNPS | 7 (4–10) | 7 (5–9) | 0 ± 0 (−1, 1) | 0.48* |
| OV | 8 (6–11) | 6 (4–9) | 2 ± 1 (1, 3)* | 0.31* |
Data are presented as median (interquartile range) unless otherwise specified and reflect recorded values from individual ovaries rounded to the nearest whole number. The mean difference (MD) reflects the right minus the left ovary. Therefore, a positive number reflects a larger value in the right ovary.
An asterisk reflects a significant mean difference (based on Wilcoxon Signed Rank Tests) or correlation (based on Spearman’s rank coefficient) between ovaries, p < 0.05.
Abbreviations: FNPO, follicle number per ovary; FNPS, follicle number per cross-section; OV, ovarian volume; MD, mean difference; SE, standard error; CI, confidence interval.
Impact on the Ultrasonographic Diagnosis of PCOS
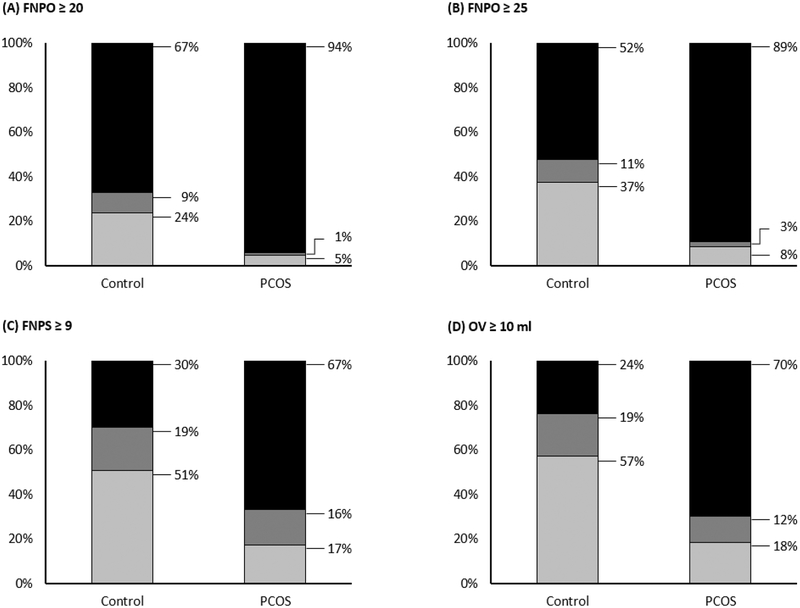
The proportion of women with uni- or bilateral PCOM, based on recent international consensus guidelines (3,7) or proposed diagnostic thresholds (13), is shown in Figure 1.
Figure 1. Proportion of women with uni- or bilateral polycystic ovarian morphology (PCOM).
Women with PCOS (n = 87) and controls (n = 67) were categorized based on the presence of PCOM in neither (light gray), one (dark gray), or both ovaries (black). PCOM was defined according to recent international consensus guidelines or proposed diagnostic thresholds including: (A) FNPO ≥ 20 (3), (B) FNPO ≥ 25 (7), (C) FNPS ≥ 9 (13), and (D) OV ≥ 10 ml (3,7,16). Abbreviations: FNPO, follicle number per ovary; FNPS, follicle number per cross-section; OV, ovarian volume.
Most women with PCOS demonstrated bilateral PCOM, regardless of the ultrasonographic marker or threshold applied (Figure 1A – 1D). FNPO represented the most consistent intra-individual marker of PCOM in PCOS; 94% of women had ≥ 20 follicles in both ovaries (Figure 1A) and 89% had ≥ 25 follicles in both ovaries (Figure 1B). Less than 4% of women with PCOS had elevated FNPO in a single ovary by either diagnostic threshold (Figure 1A – B). No differences were observed in the probability of uni- versus bilateral PCOM based on FNPO ≥ 20 (κ = 0.88; p = 0.32) or ≥ 25 (κ = 0.86; p = 0.16). FNPS and OV were less consistent intra-individual markers of PCOM in PCOS. Although two-thirds of women with PCOS demonstrated bilateral PCOM with each marker, 16% had elevated FNPS in a single ovary (Figure 1C) and 12% had elevated OV in a single ovary (Figure 1D). Differences were observed in the probability of uni- versus bilateral PCOM based on both FNPS ≥ 9 (κ = 0.59; p < 0.01) and OV ≥ 10 ml (κ = 0.70; p < 0.01).
Most women in the control group also demonstrated bilateral PCOM based on FNPO; 67% of women had ≥ 20 follicles in both ovaries (Figure 1A) and 52% had ≥ 25 follicles in both ovaries (Figure 1B). FNPO continued to represent the most consistent intra-individual marker in controls; only 9% – 11% of women had variable morphology between ovaries (Figure 1A – 1B). Fewer controls demonstrated bilateral PCOM based on FNPS ≥ 9 (30%) or OV ≥ 10 ml (24%) and 19% had elevated FNPS (Figure 1C) or elevated OV in a single ovary (Figure 1D). Such variation contributed to differences in the probability of uni- versus bilateral PCOM based on FNPO ≥ 20 (κ = 0.79; p = 0.01), FNPO ≥ 25 (κ = 0.79; p < 0.01), FNPS ≥ 9 (κ = 0.61; p < 0.01), and OV ≥ 10 ml (κ = 0.59; p < 0.01) (Figure 1).
DISCUSSION
Overall, we detected right – left differences in ultrasonographic markers of ovarian morphology – which had a significant impact on the identification of PCOM, depending on the ovary and marker considered. Discordant diagnoses of PCOM in one versus both ovaries occurred with the use of FNPS and OV, but not FNPO. The present study is timely given increasing global attention to the definition of PCOM and its implications for evidence-based guidelines to diagnose and manage PCOS (3,7). Data reported herein are unique in that we used higher frequency ultrasound transducers (7) and reliable measurement techniques (24) in well-defined cohorts.
We propose that FNPO is the most reliable intra-individual marker of PCOM. FNPO showed the strongest correlation between ovaries and there were no differences in the probability of uni- versus bilateral PCOM in women with PCOS. Our findings add to evidence that FNPO offers the greatest diagnostic potential for PCOS (3,7–14). Interestingly, one in ten controls had elevated follicle counts in a single ovary by both the International PCOS Network and Androgen Excess and PCOS Society criteria (3,7). Proximity of follicle counts to the diagnostic threshold (i.e., within 0 – 2 follicles) appeared to contribute to differences in the probability of uni- versus bilateral PCOM in controls. Therefore, we suspect that right – left differences in FNPO are most relevant to the diagnosis of PCOM in women with mild follicular excess (i.e., 15 – 30 follicles).
FNPS and OV seem to be less reliable intra-individual markers of PCOM. Both endpoints had moderate correlations between ovaries in women with PCOS, but weak correlations in controls. Discordant diagnoses of PCOM occurred when FNPS and OV were only considered in a single ovary. Previous studies have also reported weaker correlations between ovaries for OV versus FNPO (26) and suggested that size differences make PCOM more likely on the right side (27). Atiomo and colleagues proposed that the right ovary could offer differential diagnostic potential for PCOS, as a result (28). Post-hoc analyses of our data revealed that right OV (area under the receiver operating characteristic curve [AUC] = 0.78; p < 0.05) and left OV (AUC = 0.78; p < 0.05) offered similar diagnostic potential for PCOS. However, a higher threshold for OV was required in the right ovary (Threshold: 10 ml; Sensitivity: 74%; Specificity: 71%) versus the left ovary (Threshold: 9 ml; Sensitivity: 67%; Specificity: 76%) to distinguish women with PCOS from controls. We appreciate that generating side-specific diagnostic thresholds for PCOM would lead to confusion in clinical practice. Therefore, because FNPS and OV may be most prone to right – left differences, we recommend that their use be avoided if only one ovary can be assessed.
We noted that the right ovary contained more follicles than the left ovary in women with PCOS, consistent with the greater volume of the right ovary. Our findings are similar to those of some (26,27,29,30), but not all (31), previous studies of right – left differences in FNPO in women with infertility or PCOS, wherein authors have reported 0.1 – 2.0 more follicles in the right ovary (26,27,29,30). Our higher result of 5 follicles could reflect our unique approach to offline image analysis (24) compared to others’ real-time (27,29–31) or semi-automated counts (26). There are some challenges associated with the assessment of FNPO, including distinguishing an anechoic structure as one or two clustered follicles and re-counting follicles that have already been flagged. It is especially difficult to obtain reliable counts amidst the follicular density in polycystic ovaries (32,33). By evaluating each ovary in small sections, we improve reproducibility and minimize the likelihood of under- or over-counting follicles (24). Although our approach is not feasible in clinical practice due to the time required for analysis, it offers a level of accuracy that is beneficial in research settings (24). Use of the grid system may also explain why we did not detect right-sided differences in FNPO in controls (i.e., mean difference, 0 follicles) when others did (i.e., 0.4 – 1.0 follicles) (29,34).
It is important to consider the clinical relevance of intra-individual differences in follicle number. Deb et al. have reported intra-observer and inter-method variability in counts as wide as 11 follicles (35,36) – suggesting that mean differences < 11 could be within error. Small differences would also be difficult to capture in a single cross-section of the ovary (i.e., FNPS). Alternatively, evidence of more frequent natural and stimulated ovulations in the right ovary (37–39) supports the notion that ovarian reserve (and thus, folliculogenesis) is lateralized. Studies in cohorts with unspecified infertility have suggested that right – left differences in FNPO reflect an increased number of small- (27) or medium-sized (26) follicles in the right ovary. It could be speculated that aspects of follicular recruitment vary between the two ovaries. This observation has not been made in healthy women (40), but longitudinal studies could reveal the mechanisms behind right – left differences in FNPO in PCOS.
We also noted that the right ovary was larger than the left ovary in both groups. Our findings are consistent with recent studies of right – left differences in OV in women with regular cycles (34), infertility (26,27,41), and PCOS (29). Average right – left differences have ranged from 0.5 to 1.6 ml (26,27,29,34,41), with one study reporting an upper limit of agreement near 5.0 ml (26). Evidence that the right ovary is larger than the left ovary across the lifespan, from childhood (42) through the fourth decade (27,29) and post-mortem (43), raises our confidence in our findings. Differences in blood supply between the two ovaries could offer a physiologic basis for one being larger – albeit studies employing Doppler ultrasound technology have yielded conflicting data on right – left differences in vascular indices, independent of gravidity or parity (26,34,41,44,45).
An unexpected finding was that 52% – 67% of controls had bilateral PCOM based on modern diagnostic thresholds for FNPO. Our observation is reminiscent of concerns that emerged following publication of the Rotterdam criteria in 2003, when a heightened prevalence of polycystic ovaries was described in healthy women of reproductive age (46). We acknowledge that use of the grid system (24) could lead to increased detection of smaller follicles (e.g., 2 – 3 mm) – to an extent that may limit the external validity of our results. When we excluded 2 – 3 mm follicles from control estimates of FNPO, the median value fell from 25 to 14 follicles and fewer controls had PCOM based on FNPO ≥ 20 (16%) or ≥ 25 (3%) (data not shown). We agree that standardized methods and training are needed to obviate discrepancies in follicle counts among studies and in clinical practice (3,32,33). Such efforts could be de-prioritized if more reliable assays are developed and surrogate markers of PCOM (e.g., anti-müllerian hormone) are deemed effective replacements for FNPO (3,7). It should be stated that PCOS cannot be diagnosed with a single feature (1–3) and it is unclear whether PCOM confers health risks independent of anovulation or androgen excess. Nevertheless, PCOM increases the likelihood of ovarian hyperstimulation syndrome during gonadotropin therapy and has implications for success of treatment (47). Studies that characterize the physiologic range of FNPO and OV would certainly help to clarify the significance of PCOM in women with and without PCOS.
The present study had two limitations. First, in an effort to increase our sample size, we pooled ultrasonographic data obtained at four different sites with two different brands of ultrasound machines. We employed the same acquisition protocols across sites and relied on newer technology (i.e., as defined by a maximum transducer frequency ≥ 8 MHz). However, we appreciate that combining multiple datasets can introduce error and confound the true effect(s) of interest. We did not detect an impact of site or technology on FNPO, FNPS, or OV, but recommend that our findings be interpreted with caution, nevertheless. Second, the present study was not designed to identify the factors responsible for right – left differences in ovarian morphology. Although we accounted for the potential influence of obesity, gravidity, and parity, we acknowledge that the ovaries are more difficult to visualize in women with severe obesity and that our inclusion criteria may have skewed our assessments to leaner cohorts. Likewise, our use of the NIH criteria precluded us from evaluating any role of phenotype. Use of the Rotterdam criteria in future studies would help to elucidate the effect(s) of anovulation and hyperandrogenism on right –left differences in FNPO, FNPS, and OV.
In conclusion, significant intra-individual differences were observed in ultrasonographic markers of ovarian morphology among controls and women with PCOS. FNPO showed the smallest differences between ovaries. Our data may be interpreted to mean that PCOM can be reliably diagnosed in a single ovary using FNPO, but not FNPS or OV. We recommend that practitioners and researchers acknowledge the potential for right – left differences in ultrasonographic markers of ovarian morphology and use FNPO to define PCOM when only one ovary can be visualized.
ACKNOWLEDGEMENTS
This study was funded by the Division of Nutritional Sciences at Cornell University, in addition to the President’s Council of Cornell Women, Saskatchewan Health Research Foundation, Canadian Institutes of Health Research, United States Department of Agriculture (Grant 2010–34324-20769), and National Institutes of Health (Grants R56-HD089962 and ULTR00457). BYJ and HVB also received training awards from the National Institutes of Health (Grant T32-DK007158) and Canadian Institutes of Health Research (Funding Reference Number 146182).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–7. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale H, Futterweit W, et al. Position Statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–45. [DOI] [PubMed] [Google Scholar]
- 3.Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughesdon P. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.” Obstet Gynecol Surv 1982;37:59–77. [DOI] [PubMed] [Google Scholar]
- 5.Maciel G, Baracat E, Benda J, Markham S, Hensinger K, Chang R, et al. Stockpiling of Transitional and Classic Primary Follicles in Ovaries of Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab Metab 2004;89:5321–7. [DOI] [PubMed] [Google Scholar]
- 6.Webber L, Stubbs S, Stark J, Trew G, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet 2003;362:1017–21. [DOI] [PubMed] [Google Scholar]
- 7.Dewailly D, Lujan M, Carmina E, Cedars M, Laven J, Norman R, et al. Definition and significance of polycystic ovarian morphology: A task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update 2014;20:334–52. [DOI] [PubMed] [Google Scholar]
- 8.Jonard S, Robert Y, Dewailly D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod 2005;20:2893–8. [DOI] [PubMed] [Google Scholar]
- 9.Allemand M, Tummon I, Phy J, Foong S, Dumesic D, Session D. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril 2006;85:214–9. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Li L, Chen X, Zhang Q, Wang W, Li Y, et al. Ovarian volume and follicle number in the diagnosis of polycystic ovary syndrome in Chinese women. Ultrasound Obstet Gynecol 2008;32:700–3. [DOI] [PubMed] [Google Scholar]
- 11.Köşüş N, Köşüş A, Turhan N, Kamalak Z. Do threshold values of ovarian volume and follicle number for diagnosing polycystic ovarian syndrome in Turkish women differ from western countries? Eur J Obstet Gynecol Reprod Biol 2011;154:177–81. [DOI] [PubMed] [Google Scholar]
- 12.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod 2011;26:3123–9. [DOI] [PubMed] [Google Scholar]
- 13.Lujan M, Jarrett B, Brooks E, Reines J, Peppin A, Muhn N, et al. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod 2013;28:1361–8. [DOI] [PubMed] [Google Scholar]
- 14.Christ J, Willis A, Brooks E, Vanden Brink H, Jarrett B, Pierson R, et al. Follicle number, not assessments of the ovarian stroma, represents the best ultrasonographic marker of polycystic ovary syndrome. Fertil Steril 2014;101:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod 2003;18:598–603. [DOI] [PubMed] [Google Scholar]
- 16.Balen A, Laven J, Tan S, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 2003;9:505–14. [DOI] [PubMed] [Google Scholar]
- 17.Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181–91. [Google Scholar]
- 18.Lujan M, Bloski T, Chizen D, Lehotay D, Pierson R. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod 2010;25:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christ J, Vanden Brink H, Brooks E, Pierson R, Chizen D, Lujan M. Ultrasound features of polycystic ovaries relate to degree of reproductive and metabolic disturbance in polycystic ovary syndrome. Fertil Steril 2015;103:787–94. [DOI] [PubMed] [Google Scholar]
- 20.Clark N, Podolski A, Brooks E, Chizen D, Pierson R, Lehotay D, et al. Prevalence of Polycystic Ovary Syndrome Phenotypes Using Updated Criteria for Polycystic Ovarian Morphology: An Assessment of Over 100 Consecutive Women Self-reporting Features of Polycystic Ovary Syndrome. Reprod Sci 2014;21:1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanden Brink H, Willis A, Jarrett B, Lin A, Soler S, Best S, et al. Sonographic markers of ovarian morphology, but not hirsutism indices, predict serum total testosterone in women with regular menstrual cycles. Fertil Steril 2016;105:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zawadzki J, Dunaif A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach In: Dunaif A, Givens J, Haseltine F, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. p. 377–84. [Google Scholar]
- 23.Yildiz B, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2009;16:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lujan M, Brooks E, Kepley A, Chizen D, Pierson R, Peppin A. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol 2010;36:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosner W, Auchus R, Azziz R, Sluss P, Raff H. Position statement: Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. J Clin Endocrinol Metab 2007;92:405–13. [DOI] [PubMed] [Google Scholar]
- 26.Deb S, Kannamannadiar J, Campbell B, Clewes J, Raine-Fenning N. The interovarian variation in three-dimensional ultrasound markers of ovarian reserve in women undergoing baseline investigation for subfertility. Fertil Steril 2011;95:667–72. [DOI] [PubMed] [Google Scholar]
- 27.Korsholm A-S, Hvidman H, Bentzen J, Andersen A, Petersen K. Left–right differences in ovarian volume and antral follicle count in 1423 women of reproductive age. Gynecol Endocrinol 2016; Available from: https://www.tandfonline.com/doi/full/10.1080/09513590.2016.1259406. [DOI] [PubMed] [Google Scholar]
- 28.Atiomo W, Pearson S, Shaw S, Prentice A, Dubbins P. Ultrasound criteria in the diagnosis of polycystic ovary syndrome (PCOS). Ultrasound Med Biol 2000;26:977–80. [DOI] [PubMed] [Google Scholar]
- 29.Köninger A, Koch L, Edimiris P, Nießen S, Kasimir-Bauer S, Kimmig R, et al. Intraindividual right–left comparison of sonographic features in polycystic ovary syndrome (PCOS) diagnosis. Eur J Obstet Gynecol 2014;181:124–9. [DOI] [PubMed] [Google Scholar]
- 30.Alserri A, Kuriya A, Holzer H, Tulandi T. Lateralization of ovarian follicles. Gynecol Obstet Invest 2014;77:117–20. [DOI] [PubMed] [Google Scholar]
- 31.Pache T, Wladimiroff J, Hop W, Fauser B. How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology 1992;183:421–3. [DOI] [PubMed] [Google Scholar]
- 32.Lujan M, Chizen D, Peppin A, Kriegler S, Leswick D, Bloski T, et al. Improving inter-observer variability in the evaluation of ultrasonographic features of polycystic ovaries. Reprod Biol Endocrinol 2008;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lujan M, Chizen D, Peppin A, Dhir A, Pierson R. Assessment of ultrasonographic features of polycystic ovaries is associated with modest levels of inter-observer agreement. J Ovarian Res 2009;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jokubkiene L, Sladkevicius P, Valentin L. Number of Antral Follicles, Ovarian Volume, and Vascular Indices in Asymptomatic Women 20 to 39 Years Old as Assessed by 3-Dimensional Sonography. J Ultrasound Med 2012;31:1635–49. [DOI] [PubMed] [Google Scholar]
- 35.Deb S, Campbell B, Clewes J, Raine-Fenning N. Quantitative analysis of antral follicle number and size: a comparison of two-dimensional and automated three-dimensional ultrasound techniques. Ultrasound Obstet Gynecol 2010;35:354–60. [DOI] [PubMed] [Google Scholar]
- 36.Deb S, Jayaprakasan K, Campbell B, Clewes J, Johnson I, Raine-Fenning N. Intraobserver and interobserver reliability of automated antral follicle counts made using three-dimensional ultrasound and SonoAVC. Ultrasound Obstet Gynecol 2009;33:477–83. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda M, Fukuda K, Yding Andersen C, Byskov AG. Does corpus luteum locally affect follicular growth negatively? Hum Reprod 1997;12:1024–7. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda M, Fukuda K, Yding Andersen C, Byskov A. Right-sided ovulation favours pregnancy more than left-sided ovulation. Hum Reprod 2000;15:1921–6. [DOI] [PubMed] [Google Scholar]
- 39.Lan K, Huang F, Lin Y, Kung F, Lan T, Chang S. Significantly superior response in the right ovary compared with the left ovary after stimulation with follicle-stimulating hormone in a pituitary down-regulation regimen. Fertil Steril 2010;93:2269–73. [DOI] [PubMed] [Google Scholar]
- 40.Baerwald A, Adams G, Pierson R. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003;80:116–22. [DOI] [PubMed] [Google Scholar]
- 41.Jarvela I, Mason H, Sladkevicius P, Kelly S, Ojha K, Campbell S, et al. Characterization of Normal and Polycystic Ovaries Using Three-Dimensional Power Doppler Ultrasonography. J Assist Reprod Genet 2002;19:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin I, Cole T, Duncan K, Hollman A, Donaldson M. Pelvic ultrasound measurements in normal girls. Acta Pædiatrica 1995;84:536–43. [DOI] [PubMed] [Google Scholar]
- 43.Perven H, Nurunnabi A, Ara S, Jahan M. Cadaver study of the volume of the ovary in Bangladeshi women. Bangladesh Med Res Counc Bull 2014;40:15–8. [DOI] [PubMed] [Google Scholar]
- 44.Pan H, Wu M, Cheng Y, Li C, Chang F. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: a possible new marker for diagnosis. Hum Reprod 2002;17:201–6. [DOI] [PubMed] [Google Scholar]
- 45.Wu M, Tang H, Hsu C, Wang S, Huang K. The role of three-dimensional ultrasonographic images in ovarian measurement. Fertil Steril 1998;69:1152–5. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone E, Rosen M, Neril R, Trevithick D, Sternfeld B, Murphy R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab 2010;95:4965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendriks D, Mol B, Bancsi L, Te Velde E, Broekmans F. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril 2005;83:291–301. [DOI] [PubMed] [Google Scholar]