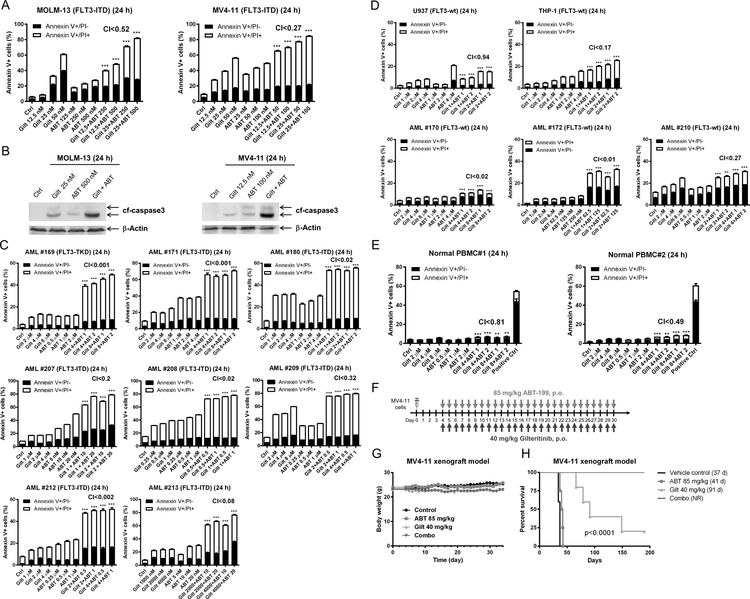
Figure 5. Gilteritinib enhances venetoclax activity both in vitro and in vivo.
(A) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone or in combination, for 24 hours. Annexin V-FITC/PI staining and flow cytometry analyses results are shown. ***indicates p<0.001. (B) MOLM-13 and MV4–11 cells were treated as indicated for 24 hours. Whole cell lysates were subjected to Western blotting. (C-E) Primary AML patient samples (panels C&D) and normal peripheral blood mononuclear cells (PBMC; panel E) were treated with gilteritinib and venetoclax, alone or in combination, for 24 hours. Annexin V-FITC/PI staining and flow cytometry analyses results are shown. ***indicates p<0.001. (F-H) MV4–11 cells (1 x 106 cells/mouse) were injected through the tail vein of immunocompromised NSGS mice. Four days post cell injection the mice were randomized (5 mice/group) and treated daily with vehicle control (3% 200 proof ethanol, 1% polyoxyethylene (20) sorbitan monooleate, and USP water), 40 mg/kg gilteritinib (p.o.), 85 mg/kg venetoclax (p.o.), or 40 mg/kg gilteritinib + 85 mg/kg venetoclax (p.o.) for 27 consecutive days (treatments were stopped when one mouse in the vehicle control group showed leukemic symptoms; panel F). Body weights were measured on a daily basis and are graphed as mean ± SEM (panel G). Overall survival probability, estimated with the Kaplan-Meier method, is shown (panel H).