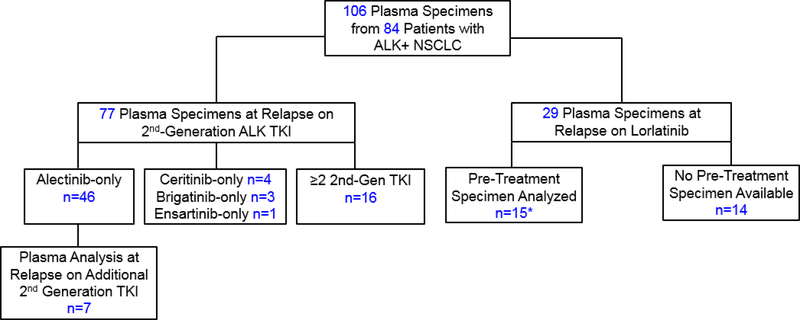
Figure 1. Study Population.
Serial analysis of plasma specimens was performed for 22 patients (7 patients who received a second-generation TKI followed by second-generation TKI, and 15 patients who received a second-generation TKI followed by lorlatinib, indicated by asterisk). Among 16 patients who received ≥2 second-generation ALK TKIs before plasma analysis, 9 received alectinib and ceritinib, 4 received alectinib and brigatinib, 2 received alectinib, ceritinib, and brigatinib, and 1 received alectinib, brigatinib, and ensartinib. *Included in both second-generation and lorlatinib cohorts; 2nd-Gen=second-generation; TKI: tyrosine kinase inhibitor.