Abstract
Interaction between tocopherol homologs and peppermint extract added to oil-in-water emulsions was studied during iron-catalyzed oxidation. Emulsions consisted of tocopherol-stripped soybean oil and citrate buffer (4:6, w/w) with/without addition of peppermint extract (400 mg/kg) and α-, γ-, or δ-tocopherol (600 mg/kg), and were oxidized in the iron presence at 25 °C. Lipid oxidation of emulsions was evaluated based on hydroperoxide contents and p-anisidine values. Lipid oxidative stability of emulsions was improved by added peppermint extract, and co-added γ- and δ-tocopherols further reduced lipid oxidation, however, α-tocopherol increased it. Tocopherol contents did not change during oxidation. Polyphenol degradation in the emulsion with added peppermint extract was lower and slower by γ- and δ-tocopherols, however, α-tocopherol showed opposite results. The results suggest that co-addition of tocopherols to the emulsion containing peppermint extract shift a major role of polyphenols as antioxidants from scavenging lipid (peroxy) radicals to tocopherol radical scavenging.
Keywords: Tocopherol homolog, Peppermint extract, Interaction, Lipid oxidation, Oil-in-water emulsion
Introduction
Polyphenols in plants are secondary metabolites playing an important role in the defense against multiple stressors (Ferdinando et al., 2014). Composition of polyphenols in plants is different depending on the species, and some compounds may be distributed in specialized cells or organs (Smetanska, 2018). These phenolic compounds significantly contribute to antioxidant activity to the human diet, resulting in diminished oxidative stress and prevention of chronic diseases or cancer (Pandey and Rizvi, 2009). In foods, polyphenols contribute to the bitterness, astringency, color, flavor, odor, and oxidative stability (Pandey and Rizvi, 2009), and scientific and industrial interests on them continuously have increased.
Ethanol extracts of peppermint and basil contained high amount of polyphenols (> 40 g/kg) and improved the lipid oxidative stability of a soybean oil-in-water emulsion (Kim and Choe, 2016) through iron-chelating and radical scavenging (Kim et al., 2017). α-Tocopherol, rosmarinic acid, and caffeic acid at natural concentration present in the peppermint extract (400 mg/kg) acted as antioxidants in the iron-catalyzed oxidation of acidic soybean oil-in-water emulsion, with the highest contribution by α-tocopherol despite low concentration of < 1 mg/kg (Lee and Choe, 2018). Interestingly, the improvement of the lipid oxidative stability by the addition of peppermint extract was disappeared in the emulsions which have tocopherols (534 mg/kg) derived from soybean oil (Choe and Kim, 2018), suggesting an interaction between peppermint extracts and tocopherols. Tocopherols detected in edible oils are usually a mixture of α-, γ-, and δ- homologs whose antioxidant activities are different (Choe, 2017; Yanishlieva et al., 2002), and the interactions of tocopherols with peppermint extracts may be different depending on their homologs. This study evaluated the interaction between tocopherol homologs and peppermint extract, mainly polyphenols, in the iron-catalyzed lipid oxidation of acidic soybean oil-in-water emulsion, with elucidation of the action mechanism involved.
Materials and methods
Materials
Soybean oil was a product of Samyang Corp. (Seoul, Korea) and tocopherols in the oil were completely removed using alumina column chromatography (Kim and Choe, 2016) by passing the oil through a glass column packed with silicic acid and aluminum oxide (Sigma-Aldrich Co., St. Louis, MO, USA). Dried peppermint (Mentha × piperita) was a product of the Florapharm (Schesslitz, Germany).
Preparation of emulsions and their oxidation
Emulsions were prepared with tocopherol-stripped soybean oil (400 g) and citrate buffer solution (pH 4.0, 600 g) according to the method of Kim and Choe (2016). Peppermint extract was obtained using 75% ethanol; dried peppermint, roughly ground in an Essence HR 2084 blender (Philips, Amsterdam, Netherlands), was mixed with 75% ethanol (1:10, w/v) at 25 °C and 120 rpm for 12 h, and filtered, with a final solvent removal using a rotary evaporator (N–N series; Eyela, Tokyo, Japan) at 65 °C. Ferrous sulfate (5 mg/kg; Junsei Co., Tokyo, Japan), peppermint extract (400 mg/kg), xanthan gum (350 mg/kg; Sigma-Aldrich Co.), α-, γ-, or δ- tocopherol (600 mg/kg; Sigma-Aldrich Co.), egg yolk lecithin (350 mg/kg; Goshen Biotech, Namyangju, Korea), and soybean oil were added to the citrate buffer solution in the order listed. The emulsion was finally prepared by homogenizing the mixture in an Ultra-Turrax T25 homogenizer (IKA Instruments, Staufen, Germany) for 6 min at 10,000 rpm.
The emulsion (10 g) was transferred into 20 mL serum bottles, which were then tightly capped with Teflon-coated septa (Cronus, Glocester, England) and aluminum caps. All samples prepared in duplicates were placed in an LBI-250 incubator (Daihan Labtech Co., Seoul, Korea) at 25 °C for 6 days in the dark. Light was excluded throughout the experiments.
Analysis of lipid oxidation of emulsions
Lipid oxidation of emulsions was evaluated based on the hydroperoxide contents and p-anisidine values as indicators for primary and secondary oxidation product generation, respectively. The hydroperoxide contents were determined by the ferric thiocyanate method (Kim and Choe, 2016) using a UV–visible spectrophotometer (UV-2700, Shimadzu Corp., Kyoto, Japan), and was expressed as cumene hydroperoxide (CuOOH; Sigma-Aldrich Co.). The p-anisidine value was determined spectrophotometrically according to the AOCS method Cd 18-90 (AOCS, 2006) after phase separation of emulsions (Min et al., 2003) and the instrument used was a UV-2700 spectrophotometer (Shimadzu Corp.) at 350 nm.
Determination of tocopherol and polyphenol contents
Tocopherol contents of emulsions were determined by the HPLC (Lee and Choe, 2018) after phase separation. The instrument was a YL 9100 HPLC (Younglin Instrument Co., Ltd., Anyang, Korea) equipped with a μ-porasil column (3.9 mm × 330 mm, 10 μm; Waters Co., Milford, MA, USA) and fluorescence detector at 290 and 330 nm for excitation and emission, respectively. An eluting solvent was 0.2% propan-2-ol (Mallinckrodt Baker Co., Phillipsburg, NJ, USA) in hexane (Samchun Chemical Co., Seoul, Korea) at 2.0 mL/min. Standard α-, γ-, and δ-tocopherols were used for the quantification (r2 > 0.993). Polyphenol contents were determined by the Folin–Ciocalteu method (Kim and Choe, 2017) using a spectrophotometer (UV-2700, Shimadzu Corp.) at 725 nm after the reaction of the Folin–Ciocalteu reagent (Sigma-Aldrich Co.) with the aqueous layer of an emulsion dissolved in methanol–water mixture (3:2, v/v). The content was expressed as rosmarinic acid (Sigma-Aldrich Co.) equivalents with a calibration curve (r2 = 0.999).
Statistical analysis
Data were presented as means with standard deviations, and statistically analyzed using SAS/PC (SAS 9.2, SAS Institute Inc., Cary, NC, USA) including regression analyses and Duncan’s multiple range test. The differences among data were considered as significant at 5% level.
Results and discussion
Lipid oxidation of emulsions
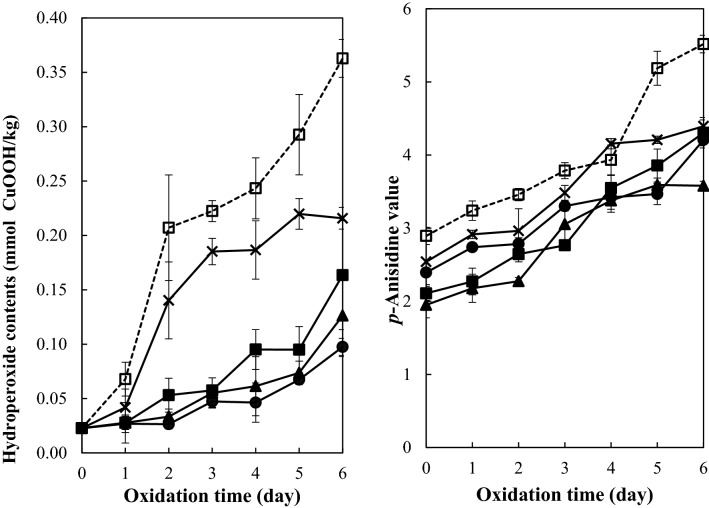
Hydroperoxide contents and p-anisidine values of emulsions with/without added peppermint extract (400 mg/kg) and one of tocopherol homologs (600 mg/kg) during iron-catalyzed oxidation at 25 °C in the dark are shown in Fig. 1. Hydroperoxide contents and p-anisidine values of the emulsions increased as the oxidation time increased, clearly indicating an occurrence of lipid oxidation. Hydroperoxide contents and p-anisidine values were significantly lower in the emulsions with added peppermint extract than in the control emulsions without the peppermint extract (p < 0.05). These results confirmed the antioxidant activity of the peppermint extract in the iron-catalyzed autoxidation of the soybean oil-in-water emulsion. The antioxidant activity of the peppermint extract was reported to be due to radical-scavenging and iron-chelating by polyphenols at high content, and rosmarinic acid was the most important radical scavenger among them (Kim and Choe, 2018).
Fig. 1.
Effect of tocopherol homolog addition (600 mg/kg) on the lipid oxidation of soybean oil-in-water emulsion (4:6, w/w) with added peppermint extract (400 mg/kg) during iron-catalyzed oxidation at 25 °C (square; control—no peppermint extract and no tocopherol, filled square; peppermint extract only, cross mark; peppermint extract + α-tocopherol, filled triangle; peppermint extract + γ-tocopherol, filled circle; peppermint extract + δ-tocopherol)
Co-addition of tocopherols to the emulsions having added peppermint extract affected the lipid oxidation differently depending on tocopherol homologs added. Hydroperoxide contents of the emulsions with peppermint extract became lower by co-addition of γ- or δ-tocopherol during 6 day oxidation; however, co-addition of α-tocopherol showed an opposite result. This suggests that α-tocopherol could increase the hydroperoxide production in the emulsion containing peppermint extract. The p-anisidine values showed a similar tendency to the hydroperoxide contents; the emulsion with added peppermint extract and α-tocopherol showed significantly higher p-anisidine values than the emulsions with only peppermint extract for 6 days (p < 0.05). This suggests that α-tocopherol could also increase the aldehyde compound production in the emulsion containing peppermint extract, while γ- and δ-tocopherols decrease it. Hydroperoxides, primary products of lipid oxidation, produce aldehyde compounds upon decomposition (Choe and Min, 2006).
Hydroperoxide contents and p-anisidine values of the emulsions showed good correlations with the oxidation time (r2 > 0.86) as shown in Table 1. The hydroperoxide and p-anisidine value increasing rates of the control emulsion which did not have added peppermint extract were 0.054 mmol/kg/day and 0.437/day, respectively, during 6 day oxidation. The emulsion with added peppermint extract showed significantly (p < 0.05) slower hydroperoxide production (0.021 mmol/kg/day) than the control emulsion, with a tendency of slower decomposition of hydroperoxides (0.381/day). Among emulsions with added peppermint extract, the emulsions with co-added γ- or δ-tocopherol showed significantly (p < 0.05) low rates of hydroperoxide production (0.015 and 0.012 mmol/kg/day, respectively) and decomposition (0.315 and 0.269/day, respectively). On the other hand, co-addition of α-tocopherol to the emulsion with added peppermint extract significantly increased the hydroperoxide production rate to 0.035 mmol/kg/day although the p-anisidine value increasing rate was not significantly different from that of the emulsion with peppermint extract only (p > 0.05). These results indicated that γ- and δ-tocopherols decelerated the lipid oxidation of the soybean oil-in-water emulsion in the co-presence of peppermint extract; however, α-tocopherol increased it.
Table 1.
Effect of tocopherol homologs (600 mg/kg) added to soybean oil-in-water emulsion (4:6, w/w) containing peppermint extract (400 mg/kg) on the regression analysis1 between time and the iron-catalyzed lipid oxidation at 25 °C for 6 days
| Additives | Hydroperoxide content (mmol CuOOH/kg) | P-Anisidine value | ||||
|---|---|---|---|---|---|---|
| A | B | R2 | A | B | R2 | |
| Control (No peppermint, no tocopherol) | 0.054a2 | 0.041 | 0.938 | 0.437a2 | 2.69 | 0.914 |
| Peppermint only | 0.021c | 0.009 | 0.891 | 0.381a | 1.92 | 0.965 |
| Peppermint + α-tocopherol | 0.035b | 0.040 | 0.869 | 0.334ab | 2.52 | 0.950 |
| Peppermint + γ-tocopherol | 0.015de | 0.011 | 0.861 | 0.315bc | 1.91 | 0.928 |
| Peppermint + δ-tocopherol | 0.012e | 0.013 | 0.862 | 0.269 cd | 2.38 | 0.930 |
1Hydroperoxide content (mmol CuOOH/kg) or p-anisidine value = a × oxidation time (day) + b, r2 = determination coefficient
2Different superscript means a significant difference among samples in the same column by dummy regression analysis (p < 0.05)
All of the above results clearly suggest that the antioxidant activity of the peppermint extract can be improved by γ- and δ-tocopherols during iron-catalyzed lipid oxidation of the emulsion; however, α-tocopherol decreased it. Negative synergisms between α-tocopherol (0.01%) and rosemary extract (0.02%) (Hraš et al., 2000) and between α-tocopherol and methanol extract of oregano, thyme, rosemary, and sage (Banias et al., 1992) were reported in the autoxidation of sunflower oil and lard, respectively. α-Tocopherol at high concentration accelerates the lipid oxidation by reducing transition metals to produce reactive hydroxyl radicals (Mahoney et al., 1984) and/or by reacting with secondary antioxidants for the recycling from tocopherol radicals to tocopherols (Bakır et al., 2013; Pyo et al., 1990). α-Tocopherol was also reported to modify the oxidation pathway and affect the generation and composition of oxidation products; α-tocopherol induced the generation of oxygenated α,β-unsaturated aldehydes and monoepoxides derived from linoleates, resulting in increased lipid oxidation (Martin-Rubio et al., 2018).
Tocopherol content changes during oxidation of emulsions
There was no tocopherol detected in the control emulsion which did not have added peppermint extract. This was due to complete stripping of tocopherols from commercially available soybean oil by alumina column chromatography before preparation of the emulsions. Total tocopherol content of the emulsion with only added peppermint extract was 0.79 mg/kg before oxidation, which might be due to natural presence of tocopherols in the peppermint extract. Detection of α- and γ-tocopherols in the ethanol extract of peppermints was reported at 1088 and 236 mg/kg, respectively (Lee and Choe, 2018). When α-, γ-, or δ-tocopherol was co-added, total contents of tocopherols of the emulsion with added peppermint extract were 593, 432, and 497 mg/kg, respectively, before oxidation (Table 2). Detection of tocopherols at different concentrations among emulsions in spite of the same addition level to the emulsion (600 mg/kg) could be due to the solubility difference among tocopherol homologs in hexane to dissolve the oil phase of emulsions for the anlaysis in this study. α-Tocopherol has more methyl group than γ- or δ-tocopherol, which makes α-tocopherol more hydrophobic (Du and Ahn, 2002) and thus more soluble in hexane. Tocopherol contents of the emulsions with added peppermint extract and tocopherols were not significantly changed during 6 day oxidation (p > 0.05). This indicated no degradation or no net loss of tocopherols in the emulsions with added peppermint extract during iron-catalyzed lipid oxidation. Tocopherols are good radical scavengers, and produce tocopherol radicals upon hydrogen donation to lipid (peroxy) radicals (Choe and Min, 2009; Yamauchi, et al., 1990), and thus no significant tocopherol content change during the emulsion oxidation suggests an occurrence of tocopherol regeneration from tocopherol radicals by some compounds in the emulsion.
Table 2.
Tocopherol contents of soybean oil-in-water emulsion (4:6, w/w) with added peppermint extract and different tocopherol homologs during iron-catalyzed oxidation at 25 °C
| Additives | 0 day | 3 days | 6 days |
|---|---|---|---|
| Peppermint extract | 0.788 ± 0.076a1 | 0.624 ± 0.040a | 0.564 ± 0.069a |
| Peppermint + α-tocopherol | 593 ± 29.7a | 590 ± 9.48a | 605 ± 6.24a |
| Peppermint + γ-tocopherol | 432 ± 34.8a | 456 ± 21.4a | 448 ± 10.3a |
| Peppermint + δ-tocopherol | 497 ± 3.62a | 487 ± 38.9a | 473 ± 0.330a |
1The same superscript means no significant difference in the same row by Duncan’s multiple range test at 5%
Polyphenol content changes during oxidation of emulsions
Polyphenols are major antioxidants with high concentration in the peppermint extract, and thus their contents were determined during the emulsion oxidation. There was no polyphenol detected in the control emulsion to which the peppermint extract was not added since polyphenols in the emulsions were derived from the peppermint extract. Rosmarinic acid, isosalvianolic acid A, and salvianolic acid B were reported as predominant (> 60%) polyphenols in the ethanol extract of peppermint (Kim and Choe, 2018). Polyphenol contents of the emulsions with added peppermint extract were in the range of 50.1–51.7 mg/kg before oxidation and decreased during the oxidation (Fig. 2), indicating its degradation. The emulsions with added peppermint extract and α-tocopherol showed the lowest polyphenol content during 6 day oxidation, and the emulsions with both peppermint extract and δ-tocopherol tended to show higher polyphenol contents than the emulsion with added peppermint extract only. This indicated that co-addition of α-tocopherol increased degradation of polyphenols in the emulsion with added peppermint extract during the iron-catalyzed oxidation; however, polyphenol degradation was decreased in the co-addition of δ-tocopherol. Degradation of polyphenols during lipid oxidation is more directly related with their role in radical scavenging than iron-chelation (Kim and Choe, 2017; Kim and Choe, 2018). When polyphenols form a complex with iron (Fe2+-polyphenol) and then oxidation occurs to Fe3+-polyphenol complex, oxygen is transferred to less reactive oxygen species instead of polyphenols, resulting in no degradation of polyphenols (Perron and Brumaghim, 2009). Thus significant degradation of polyphenols suggests their action as radical scavengers during iron-catalyzed oxidation of the emulsions with co-added tocopherol homologs. This, in turn, suggests that α-tocopherol co-added to the emulsions could increase radical scavenging action of polyphenols, however, δ-tocopherol decreased it.
Fig. 2.
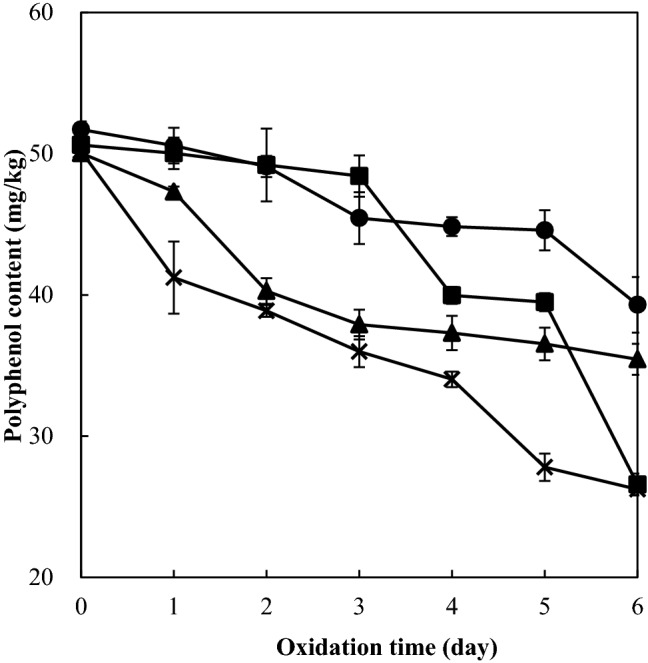
Polyphenol content of soybean oil-in-water emulsion (4:6, w/w) with added peppermint extract (400 mg/kg) and different tocopherol homologs (600 mg/kg) during iron-catalyzed oxidation at 25 °C (filled square; peppermint extract only, cross mark; peppermint extract + α-tocopherol, filled triangle; peppermint extract + γ-tocopherol, filled circle; peppermint extract + δ-tocopherol)
Degradation of polyphenols was highly correlated with time (r2 > 0.75) as shown in Table 3. Degradation of polyphenols derived from the peppermint extract was significantly (p < 0.05) slowed down by co-addition of γ- or δ-tocopherol (0.058 and 0.042 mg/kg/day, respectively) compared to the emulsion with the peppermint extract only (0.093 mg/kg/day). The degradation rate of polyphenols in the emulsion with both peppermint extract and α-tocopherol was not significantly different from that in the emulsion with peppermint extract only (p > 0.05) although there was a tendency of increase. These results clearly indicate that γ- and δ-tocopherols decelerated polyphenol degradation with an accelerating tendency by α-tocopherol in the emulsion with added peppermint extract during iron-catalyzed oxidation.
Table 3.
Effect of tocopherol homologs (600 mg/kg) added to soybean oil-in-water emulsion (4:6, w/w) containing peppermint extract (400 mg/kg) on the polyphenol degradation1 during iron-catalyzed lipid oxidation at 25 °C for 6 days
| Additives | A | B | R2 |
|---|---|---|---|
| Peppermint | 0.093a2 | 56.3 | 0.750 |
| Peppermint + α-tocopherol | 0.102a | 48.3 | 0.967 |
| Peppermint + γ-tocopherol | 0.058b | 48.1 | 0.874 |
| Peppermint + δ-tocopherol | 0.042c | 52.5 | 0.915 |
1Ln (Polyphenol retention, % based on the content at zero time) = − a × oxidation time (day) + b, r2 = determination coefficient
2Different letters represent significant difference in the degradation rate among emulsions by dummy variable regression analysis at 5%
Considering no degradation of tocopherols during 6 day oxidation of the emulsions with added peppermint extract and/or tocopherol homologs, higher and faster degradation of polyphenols by co-added α-tocopherol and slower degradation by γ- or δ-tocopherol suggest an interaction between polyphenols in the peppermint extract and tocopherols. The physical arrangement of phenol groups of tocopherols at or near the water–oil interface in the emulsion facilitates hydrophilic polyphenols to effectively inhibit the attack by free radicals in the aqueous phase and to repair tocopherol radicals to tocopherols (Laranjinha and Cadenas, 1999). It was reported that noticeable amount of gallic acid was involved in the reduction of tocopherol radical to a less reactive form (Rudolphi-Skórska et al., 2016). The antioxidant activity of caffeic acid in the low-density lipoprotein oxidation was reported to be largely related to the reduction of tocopherol radicals at the bilayer surface rather than direct interception of lipid peroxy radicals (Laranjinha and Cadenas, 1999). The bond dissociation energy between oxygen and hydrogen in a phenol group is the highest in δ-tocopherol, followed by γ- and α-tocopherols, which suggests that α-tocopherol can scavenge lipid (peroxy) radicals the most effectively (Choe and Min, 2009). Broznic et al. (2016) reported higher radical scavenging ability of α-tocopherol compared to those of γ- and δ-tocopherols. This means, in turn, that tocopherol radicals could be produced at higher concentration in the emulsion with co-added α-tocopherol than in the emulsion with γ- or δ-tocopherol. Polyphenols as a reductant in the emulsion with co-added α-tocopherol could contribute more to regeneration of tocopherols, resulting in higher degradation of polyphenols. Degradation of polyphenols involves production of oxidation compounds, which can accelerate the lipid oxidation. Unstable quinone on the 2-oxyphenylpropanoyl moiety was reported as oxidation product of rosmarinic acid (Fujimoto and Masuda, 2012), predominant polyphenol compound in the peppermint extract. Therefore, in this study, the lipid oxidation could be higher in the α-tocopherol co-added emulsion which showed higher degradation of polyphenols than in the emulsions with γ- or δ-tocopherol.
In conclusion, polyphenols in the peppermint extract decreased the lipid oxidation of soybean oil-in-water emulsion, and co-added γ- and δ-tocopherols further improved the lipid oxidative stability, but α-tocopherol reduced the antioxidant activity of the peppermint extract. Co-addition of tocopherols modified the antioxidant pathway of polyphenols derived from the peppermint extract in the emulsion; polyphenols in the emulsions without co-added tocopherols contributed to the improved lipid oxidative stability by directly scavenging on lipid (peroxy) radicals. However, under the co-addition of tocopherols to the emulsion with added peppermint extract, contribution of polyphenols as antioxidants could be mainly due to scavenging on tocopherol radicals.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A2A01053245).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jihee Kim, Email: jihi0142@daum.net.
Eunok Choe, Phone: 82-32-860-8125, Email: eochoe@inha.ac.kr.
References
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. 4th ed. Method Cd 18-90. AOCS Press, Champaign, IL, USA (2006)
- Bakır T, Beker BY, Sönmezoğlu İ, İmer F, Apak R. Antioxidant and prooxidant effects of α-tocopherol in a linoleic acid-copper(II)-ascorbate system. Eur. J. Lipid Sci. Technol. 2013;115:372–376. doi: 10.1002/ejlt.201200124. [DOI] [Google Scholar]
- Banias C, Oreopoulou V, Thomopoulos CD. The effect of primary antioxidants and synergists on the activity of plant extracts in lard. J. Am. Oil Chem. Soc. 1992;69:520–524. doi: 10.1007/BF02636101. [DOI] [Google Scholar]
- Broznić D, Jurešić GČ, Milin Č. Involvement of α-, γ- and δ-tocopherol isomers from pumpkin (Cucurbita pepo L.) seed oil or oil mixtures in the biphasic DPPH˙ disappearance kinetics. Food Technol. Biotechnol. 54: 200–210 (2016) [DOI] [PMC free article] [PubMed]
- Choe E. Effects and mechanisms of minor compounds in oil on lipid oxidation. In: Akoh CC, editor. Food Lipids: Chemistry, Nutrition, and Biotechnology. 4. Boca Raton, FL, USA: CRC Press; 2017. pp. 567–590. [Google Scholar]
- Choe E, Kim J. Effect of tocopherols present in soybean oil on the antioxidant activity of peppermint extract during autoxidation of oil-in-water emulsion. Korean J. Food Cook. Sci. 2018;34:172–177. doi: 10.9724/kfcs.2018.34.2.172. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- Du M, Ahn DU. Simultaneous analysis of tocopherols, cholesterol, and phytosterols using gas chromatography. J. Food Sci. 2002;67:1696–1700. doi: 10.1111/j.1365-2621.2002.tb08708.x. [DOI] [Google Scholar]
- Ferdinando MD, Brunetti C, Agati G, Tattini M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014;103:107–116. doi: 10.1016/j.envexpbot.2013.09.012. [DOI] [Google Scholar]
- Fujimoto A, Masuda T. Antioxidation mechanism of rosmarinic acid, identification of an unstable quinone derivative by the addition of odourless thiol. Food Chem. 2012;132:901–906. doi: 10.1016/j.foodchem.2011.11.062. [DOI] [Google Scholar]
- Hraš AR, Hadolin M, Knez Ž, Bauman D. Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000;71:229–233. doi: 10.1016/S0308-8146(00)00161-8. [DOI] [Google Scholar]
- Kim J, Choe E. Effects of selected herb extracts on iron-catalyzed lipid oxidation in soybean oil-in-water emulsion. Food Sci. Biotechnol. 2016;25:1017–1022. doi: 10.1007/s10068-016-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Choe E. Improvement of the lipid oxidative stability of soybean oil-in water emulsion by addition of daraesoon (shoot of Actinidia arguta) and samnamul (shoot of Aruncus dioicus) extract. Food Sci. Biotechnol. 2017;26:113–119. doi: 10.1007/s10068-017-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Choe E. Effect of the pH on the lipid oxidation and polyphenols of soybean oil-in-water emulsion with added peppermint (Mentha piperita) extract in the presence and absence of iron. Food Sci. Biotechnol. 2018;27:1285–1292. doi: 10.1007/s10068-018-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee H, Choe E. Effects of basil extract and iron addition on the lipid autoxidation of soybean oil-in-water emulsion with high oil content. Korean J. Food Cook. Sci. 2017;33:113–120. doi: 10.9724/kfcs.2017.33.1.113. [DOI] [Google Scholar]
- Laranjinha J, Cadenas E. Redox cycles of caffeic acid, α-tocopherol, and ascorbate: implications for protection of low-density lipoproteins against oxidation. IUBMB Life. 1999;48:57–65. doi: 10.1080/713803474. [DOI] [PubMed] [Google Scholar]
- Lee H, Choe E. Contribution of minor compounds present in the peppermint (Mentha piperita) to the iron-catalyzed lipid oxidation of soybean oil-in-water emulsion. Food Sci. Biotechnol. 2018;27:1319–1325. doi: 10.1007/s10068-018-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Graf E, Bryant RG, Eaton JW. Iron catalyzed hydroxyl radical formation. J. Biol. Chem. 1984;259:3620–3624. [PubMed] [Google Scholar]
- Martin-Rubio AS, Sopelana P, Ibargoitia ML, Guillén MD. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018;245:312–323. doi: 10.1016/j.foodchem.2017.10.098. [DOI] [PubMed] [Google Scholar]
- Min S, Mistry B, Lee HO. Improvement of oxidative and emulsion stability of model salad dressing by glucose oxidase-catalase. J. Food Sci. 2003;68:1272–1275. doi: 10.1111/j.1365-2621.2003.tb09638.x. [DOI] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Pyo YH, Ahn MS, Yim UK. Effect of tocopherols on the oxidation stability of evening primrose oil. Korean J. Food Sci. Technol. 1990;22(3):225–260. [Google Scholar]
- Rudolphi-Skórska E, Filek M, Zembala M. α-Tocopherol/gallic acid cooperation in the protection of galactolipids against ozone-induced oxidation. J. Membr. Biol. 2016;249:87–95. doi: 10.1007/s00232-015-9851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetanska I. Sustainable production of polyphenols and antioxidants by plant in vitro cultures. In: Pavlov A, Bley T, editors. Bioprocessing of Plant In Vitro Systems. Cham, Switzerland: Springer International Publishing AG; 2018. pp. 1–45. [Google Scholar]
- Yamauchi R, Matsui T, Miyake N, Kato K, Ueno Y. Reaction of δ-tocopherol with an alkylperoxyl radical. Agric. Biol. Chem. 1990;54:2993–2999. [Google Scholar]
- Yanishlieva NV, Kamal-Eldin A, Marinova EM, Toneva AG. Kinetics of antioxidant action of α- and γ -tocopherols in sunflower and soybean triacylglycerols. Eur. J. Lipid Sci. Technol. 2002;104:262–270. doi: 10.1002/1438-9312(200205)104:5<262::AID-EJLT262>3.0.CO;2-B. [DOI] [Google Scholar]