Abstract
Background
Chronic bronchitis (CB) increases risk of COPD exacerbations. We have shown that the St. George’s Respiratory Questionnaire (SGRQ) CB definition identifies patients with a similar clinical phenotype as classically defined CB. Whether the SGRQ CB definition is a predictor of future COPD exacerbations is unknown.
Methods
We analyzed 7,557 smokers with normal spirometry and Global Initiative for Chronic Obstructive Lung Disease stage 1-4 COPD in the Genetic Epidemiology of COPD study with longitudinal follow-up data on exacerbations. Subjects were divided into classic CB+ or classic CB–, using the classic definition. In addition, subjects were divided into SGRQ CB+ or SGRQ CB–. Exacerbation frequency and severe exacerbation frequency were determined in each group. Multivariable linear regressions were performed for exacerbation frequency with either classic CB or SGRQ CB and relevant covariates.
Results
There were 1,434 classic CB+ subjects and 2,290 SGRQ CB+ subjects. The classic CB+ group had a greater exacerbation frequency compared with the classic CB– group (0.69 ± 1.26 vs 0.36 ± 0.90 exacerbations per patient per year; P < .0001) and a greater severe exacerbation frequency (0.26 ± 0.74 vs 0.13 ± 0.46 severe exacerbations per patient per year; P < .0001). There were similar differences between the SGRQ CB+ and SGRQ CB– groups. In multivariable analysis, both SGRQ CB and classic CB were independent predictors of exacerbation frequency, but SGRQ CB had a higher regression coefficient. In addition, SGRQ CB was an independent predictor of severe exacerbation frequency whereas classic CB was not.
Conclusions
The SGRQ CB definition identified more subjects at risk for future exacerbations than the classic CB definition. SGRQ CB was at least a similar if not better predictor of future exacerbations than classic CB.
Key Words: cough, chronic bronchitis, COPD, exacerbations
Abbreviations: CB, chronic bronchitis; COPDGene, Genetic Epidemiology Study of Chronic Obstructive Pulmonary Disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LFU, longitudinal follow-up; mMRC, modified Medical Research Council; SGRQ, St. George’s Respiratory Questionnaire
FOR EDITORIAL COMMENT, SEE PAGE 641
COPD is a major public health problem that is the fourth leading cause of death in the United States.1 Chronic bronchitis (CB) is surprisingly common in the general population, seen in 3.4% to 22.0% of adults.2 CB hastens lung function decline, increases risk for exacerbations, reduces health-related quality of life, and raises all-cause mortality.3, 4, 5, 6, 7 However, the data on which these conclusions are based have used various definitions, including chronic phlegm, chronic mucus hypersecretion, bronchial hypersecretion, and chronic cough with phlegm.3, 8, 9, 10, 11, 12
CB is classically defined as chronic cough and sputum production for 3 months/year for at least two consecutive years.13 This definition has been the standard one for years, but many studies have used different ways to define it. The St. George’s Respiratory Questionnaire (SGRQ) has been used extensively in large COPD clinical trials, and some studies have used a definition for CB derived from the answers to the questions regarding cough and phlegm in the SGRQ.14, 15
We have shown that SGRQ CB identified 50.9% more patients with a similar clinical phenotype that is identified by the classic definition.16 In this cross-sectional analysis, history of prior exacerbations was just as great in those with classically defined CB compared with the SGRQ CB definition. The utility of the SGRQ CB definition in identifying exacerbations prospectively is not known. We hypothesized that the SGRQ CB definition would have at least a similar predictive value compared with the classic CB definition for future exacerbations.
Methods
The COPDGene (Genetic Epidemiology of COPD) study is a longitudinal study at 21 clinical centers across the United States. Subjects were non-Hispanic whites or African Americans with smoking histories of at least 10 pack-years and had no other lung disease except asthma.17 All subjects provided informed consent in writing, and the study was approved at each clinical center by their local institutional review board (full list available in e-Appendix 1). We included current or former smokers without airflow obstruction (Global Initiative for Chronic Obstructive Lung Disease [GOLD] 0) and those with COPD (GOLD 1-4). Prospective exacerbation data were captured through a longitudinal follow-up (LFU) protocol conducted every 3 to 6 months by coordinator phone calls or an automated telephonic or web-based inquiry.18 Length of follow-up varied on the basis of time of enrollment; the July 2016 LFU data set was used for this analysis.
Subjects were extensively clinically characterized, including a detailed medical history, respiratory symptoms, SGRQ, spirometry, CT scans of the chest, and 6-minute walk test. Classic CB was defined as cough and phlegm for at least 3 months per year for at least two consecutive years. The SGRQ definition of CB was defined as both cough and phlegm “almost every day” or “several days a week” over the past 4 weeks. Subjects were divided into classic CB+ vs classic CB–, or SGRQ CB+ vs SGRQ CB– separately, based on baseline data. The primary outcomes of interest were total and severe exacerbation frequencies (severe defined as requiring an ED visit or hospitalization) and percentages of the groups with total exacerbation frequency ≥ 2/year or severe exacerbation frequency ≥ 1/year. In addition, to ascertain the characteristics of subjects using both definitions, subjects were divided into four groups (classic CB–/SGRQ CB–, classic CB+/SGRQ CB–, classic CB–/SGRQ CB+, classic CB+/SGRQ CB+).
Statistics
Statistics were performed with SPSS version 24.0 (IBM Corp.). Continuous variables were compared between the two groups (classic CB+ vs classic CB– or SGRQ CB+ vs SGRQ CB–) using unpaired t tests, and categorical values were compared with χ 2 tests. When four groups were analyzed (classic CB–/SGRQ CB–, classic CB+/SGRQ CB–, classic CB–/SGRQ CB+, classic CB+/SGRQ CB+), continuous variables were analyzed by one-way analysis of variance with the Bonferroni method for multiple comparisons. Multivariable linear regression for total and severe exacerbation frequency in LFU was performed with either classic CB or SGRQ CB as the independent variables of interest with age, race, sex, pack-year history, current smoking, oxygen use, modified Medical Research Council (mMRC) dyspnea score, % emphysema, prior exacerbation frequency, FEV1% predicted, 6-minute walk distance, and body mass index as covariates. The regression coefficients for SGRQ CB and classic CB were compared with one another in these multivariable models. In addition, multivariable logistic regression was performed for exacerbation frequency ≥ 2 exacerbations/subject/year and severe exacerbation frequency ≥ 1 exacerbation/subject/year, using the same independent variables.
Results
Baseline characteristics of the groups divided by classic CB or SGRQ CB are presented in Table 1. Of the 7,557 subjects analyzed, 1,434 subjects were in the classic CB+ group (19.0% of the cohort) and 2,290 subjects (30.3% of the cohort, 59.6% more individuals than classic CB+) were in the SGRQ CB+ group. Whether divided by classic CB or SGRQ CB, those who were CB+ were more likely to be non-Hispanic white, male, and currently smoking, and had a greater pack-year history, a lower FEV1% predicted, a lower 6-minute walk distance, and higher mMRC dyspnea score. Those who were CB+ also experienced more exacerbations prior to enrollment and were more likely to have had a prior severe exacerbation. Those who were CB+ were also more likely to be treated with inhaled medications such as long-acting muscarinic antagonists, long-acting β-agonists, and inhaled corticosteroids. Breakdown of medication classes by GOLD stages is provided in e-Table 1. Those who were CB+ were also more likely to have histories of congestive heart failure and gastroesophageal reflux disease. Otherwise comorbidities between CB+ and CB– groups were not significantly different. These trends were similar whether classic CB+ or SGRQ CB+ subjects were compared with classic CB– or SGRQ CB– subjects, respectively.
Table 1.
Baseline Characteristics
| Variable | SGRQ CB– |
SGRQ CB+ |
P Value | Classic CB– |
Classic CB+ |
P Value |
|---|---|---|---|---|---|---|
| (n = 5,267) | (n = 2,290) | (n = 6,123) | (n = 1,434) | |||
| Race, non-Hispanic white, % | 71.8 | 76.5 | < .0001 | 72.2 | 77.5 | < .0001 |
| Sex, male, % | 50.3 | 57.4 | < .0001 | 51.1 | 58.5 | < .0001 |
| BMI, kg/m2 | 28.5 ± 5.9 | 28.5 ± 6.3 | .510 | 28.52 ± 5.94 | 28.46 ± 6.27 | .713 |
| Age, y | 60.86 ± 9.10 | 60.73 ± 8.80 | .548 | 60.93 ± 9.09 | 60.36 ± 8.65 | .030 |
| Smoking history, pack-years | 42.40 ± 23.58 | 50.30 ± 27.46 | < .0001 | 43.27 ± 24.24 | 51.34 ± 27.52 | < .0001 |
| Current smoking, % | 40 | 59.3 | < .0001 | 42 | 62.6 | < .0001 |
| FEV1% pred | 80.26 ± 25.94 | 67.15 ± 27.22 | < .0001 | 78.66 ± 26.52 | 66.09 ± 26.76 | < .0001 |
| FVC% pred | 90.57 ± 17.26 | 84.44 ± 19.31 | < .0001 | 89.69 ± 17.73 | 84.52 ± 19.18 | < .0001 |
| FEV1/FVC | 0.67 ± 0.16 | 0.59 ± 0.17 | < .0001 | 0.66 ± 0.16 | 0.59 ± 0.17 | < .0001 |
| 6-Min walk distance, ft | 1,412 ± 392 | 1,273 ± 397 | < .0001 | 1,395 ± 397 | 1,263 ± 387 | < .0001 |
| mMRC dyspnea score | 1.07 ± 1.34 | 1.94 ± 1.47 | < .0001 | 1.16 ± 1.38 | 2.06 ± 1.45 | < .0001 |
| Noct awake cough, % | 14.9 | 46 | < .0001 | 18.2 | 50.4 | < .0001 |
| Noct awake SOB, % | 13.7 | 33.7 | < .0001 | 15.4 | 38.4 | < .0001 |
| Allergic nasal sympt, % | 42.1 | 64.4 | < .0001 | 44.4 | 67.7 | < .0001 |
| Allergic ocular sympt, % | 37 | 51.6 | < .0001 | 38.3 | 54.6 | < .0001 |
| Dusty job ever, % | 42.2 | 54.6 | < .0001 | 43 | 58.6 | < .0001 |
| Fumes job ever, % | 43.9 | 54.7 | < .0001 | 44.4 | 59.1 | < .0001 |
| Exac freq, No./pt/y | 0.27 ± 0.75 | 0.72 ± 1.27 | < .0001 | 0.32 ± 0.83 | 0.79 ± 1.33 | < .0001 |
| History of severe exac, % | 8.3 | 19.2 | < .0001 | 9.7 | 20 | < .0001 |
| % Emphysema | 6.70 ± 9.94 | 8.44 ± 10.75 | < .0001 | 6.93 ± 10.07 | 8.50 ± 10.75 | < .0001 |
| % Gas trapping | 22.62 ± 20.06 | 28.76 ± 21.75 | < .0001 | 23.46 ± 20.48 | 28.87 ± 21.49 | < .0001 |
| Inhaled medications | ||||||
| LAMA, % | 4.3 | 7.3 | < .0001 | 4.7 | 7.6 | < .0001 |
| LABA, % | 0.5 | 1.2 | .002 | 0.6 | 1.3 | .013 |
| ICS, % | 2.6 | 4.7 | < .0001 | 2.8 | 5.3 | < .0001 |
| ICS + LABA, % | 8.2 | 14.2 | < .0001 | 8.6 | 15.9 | < .0001 |
| ICS + LAMA, % | 0.8 | 1.5 | .003 | 0.8 | 1.9 | .001 |
| LABA + LAMA, % | 0.6 | 1.5 | < .0001 | 0.8 | 1.2 | .110 |
| ICS + LABA + LAMA, % | 10.7 | 19.4 | < .0001 | 11.8 | 19.9 | < .0001 |
| Comorbidities | ||||||
| Cancer, % | 5.6 | 5.6 | 1.000 | 5.5 | 5.7 | .798 |
| Congestive heart failure, % | 2.5 | 3.9 | .001 | 2.7 | 3.9 | .019 |
| Coronary artery disease, % | 6.8 | 7.8 | .132 | 6.8 | 8.3 | .060 |
| Diabetes, % | 11.5 | 13.1 | .045 | 11.9 | 12.1 | .892 |
| Hypertension, % | 42.0 | 45.6 | .004 | 42.5 | 45.3 | .054 |
| Cerebrovascular disease, % | 2.3 | 3.1 | .055 | 2.5 | 2.4 | .925 |
| GERD, % | 25 | 31.7 | < .0001 | 24.4 | 30.4 | < .0001 |
Values are expressed as mean ± SD or percent. Medications are mutually exclusive groups. Probability values in boldface indicate significance. CB = chronic bronchitis; Exac freq = exacerbation frequency; GERD = gastroesophageal reflux disease; ICS = inhaled corticosteroids; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; mMRC = modified Medical Research Council; Noct = nocturnal; SGRQ = St. George’s Respiratory Questionnaire; SOB = shortness of breath; sympt = symptoms.
Data on COPD exacerbations during LFU are presented in Table 2. Subjects were monitored for 5.16 ± 2.06 years. Whether subjects were divided by classic CB or SGRQ CB, the CB+ group had a greater total and severe exacerbation frequency compared with the CB– group. Similarly, the percentages of subjects in the CB+ groups with a total exacerbation frequency of ≥ 2/year and severe exacerbation frequency of ≥ 1/year were higher compared with the CB– groups. These trends were also seen when subjects were separated into GOLD 0 and GOLD 1-2 disease. In those with GOLD 3-4 disease, total and severe exacerbation frequencies, as well as the percentage of those with total exacerbation frequencies of ≥ 2/year and severe exacerbation frequencies of ≥ 1/year, were greater in the SGRQ CB+ group compared with the SGRQ CB– group. However, in the classic CB+ group in those with GOLD 3-4 disease, only total exacerbation frequency was greater compared with the classic CB– group, whereas the severe exacerbation frequencies were not statistically different. Similarly, the percentages of severe exacerbation frequencies ≥ 1/year were not statistically different between the classic CB+ and classic CB– groups in GOLD 3-4 subjects.
Table 2.
Total and Severe Exacerbation Frequency in Longitudinal Follow-Up
| GOLD Stage/Exacerbation History | Classic CB Definition |
SGRQ CB Definition |
||||
|---|---|---|---|---|---|---|
| Classic CB– |
Classic CB+ |
P Value | SGRQ CB– |
SGRQ CB+ |
P Value | |
| (n = 6,121) | (n = 1,434) | (n = 5,238) | (n = 2,290) | |||
| All | ||||||
| Exac freq (No./pt/y) | 0.36 ± 0.90 | 0.69 ± 1.26 | < .0001 | 0.31 ± 0.80 | 0.69 ± 1.29 | < .0001 |
| Severe exac freq (No./pt/y) | 0.13 ± 0.46 | 0.26 ± 0.74 | < .0001 | 0.10 ± 0.39 | 0.26 ± 0.74 | < .0001 |
| Exac freq ≥ 2/y (%) | 4.7 | 10.5 | < .0001 | 3.6 | 11.0 | < .0001 |
| Severe exac freq ≥ 1/y (%) | 3.6 | 7.4 | < .0001 | 2.9 | 7.6 | < .0001 |
| GOLD stage 0 | (n = 3,160) | (n = 428) | (n = 2,847) | (n = 741) | ||
| Exac freq (No./pt/y) | 0.16 ± 0.63 | 0.30 ± 0.72 | < .0001 | 0.15 ± 0.61 | 0.30 ± 0.78 | < .0001 |
| Severe exac freq (No./pt/y) | 0.06 ± 0.34 | 0.12 ± 0.45 | < .0001 | 0.05 ± 0.30 | 0.12 ± 0.52 | < .0001 |
| Exac freq ≥ 2/y (%) | 1.6 | 3.2 | .017 | 1.4 | 3.2 | .002 |
| Severe exac freq ≥ 1/y (%) | 1.6 | 3 | .044 | 1.2 | 3.6 | < .0001 |
| GOLD stage 1-2 | (n = 1,812) | (n = 555) | (n = 1,521) | (n = 846) | ||
| Exac freq (No./pt/y) | 0.34 ± 0.74 | 0.64 ± 1.14 | < .0001 | 0.31 ± 0.67 | 0.61 ± 1.09 | < .0001 |
| Severe exac freq (No./pt/y) | 0.11 ± 0.40 | 0.23 ± 0.68 | < .0001 | 0.09 ± 0.31 | 0.22 ± 0.69 | < .0001 |
| Exac freq ≥ 2/y (%) | 4.1 | 9.4 | < .0001 | 3.3 | 9.0 | < .0001 |
| Severe exac freq ≥ 1/y (%) | 2.7 | 6.1 | < .0001 | 2.3 | 5.7 | < .0001 |
| GOLD stage 3-4 | (n = 1,124) | (n = 445) | (n = 866) | (n = 703) | ||
| Exac freq (No./pt/y) | 0.96 ± 1.39 | 1.12 ± 1.62 | .040 | 0.84 ± 1.22 | 1.21 ± 1.70 | < .0001 |
| Severe exac freq (No./pt/y) | 0.35 ± 0.71 | 0.43 ± 0.96 | .082 | 0.30 ± 0.64 | 0.46 ± 0.93 | < .0001 |
| Exac freq ≥ 2/y (%) | 14.7 | 19.1 | .039 | 11.3 | 21.6 | < .0001 |
| Severe exac freq ≥ 1/y (%) | 10.9 | 13.3 | .189 | 9.5 | 14.1 | .005 |
Data are expressed as mean ± SD or percent. Probability values in boldface indicate significance. GOLD = Global Initiative for Chronic Obstructive Lung Disease; pt = patient. See Table 1 legend for expansion of other abbreviations.
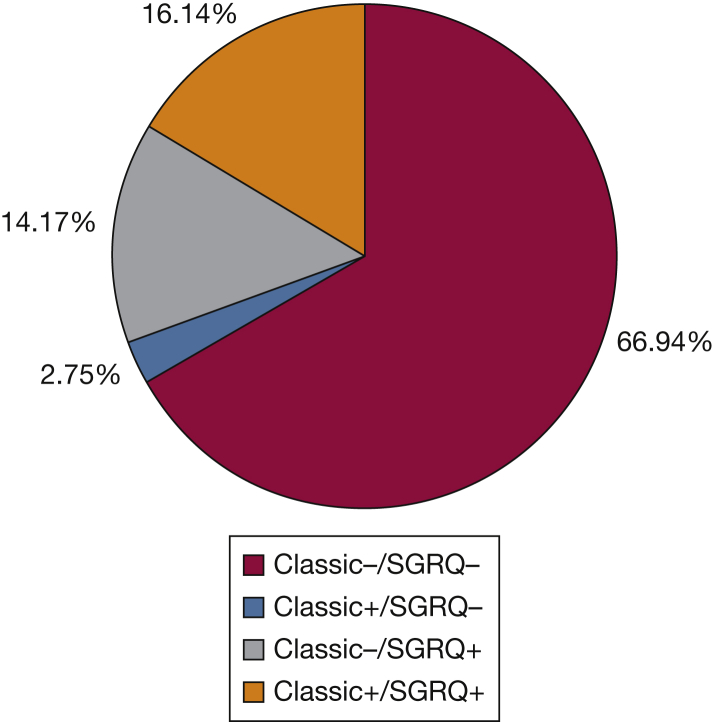
Data on the four-group analysis are presented in Table 3. Of note, there were only 208 subjects (2.75% of the cohort) in the classic CB+/SGRQ CB– group, and 1,071 subjects in the classic CB–/SGRQ CB+ group (14.17%), almost the same number as those who were classic CB+/SGRQ CB+ (n = 1,220, 16.14%). Figure 1 shows the breakdown of subjects in each group. Overall trends in differences between groups were similar when classic CB+ or SGRQ CB+ groups were compared with classic CB– or SGRQ CB– groups, respectively. Individual differences between each group are shown in Table 3. The classic CB+/SGRQ CB+ group had the worst lung function, greatest pack-year history of smoking, lowest 6-minute walk distance, highest mMRC dyspnea scores, and greatest exacerbation histories. In LFU, exacerbation frequency and severe exacerbation frequency were highest in the classic CB+/SGRQ CB+ group followed by the classic CB–/SGRQ CB+ group.
Table 3.
Baseline Characteristics and Exacerbations of Four CB Groups
| Variable | Classic CB–/SGRQ CB– |
Classic CB+/SGRQ CB– |
Classic CB–/SGRQ CB+ |
Classic CB+/SGRQ CB+ |
Overall P Value |
|---|---|---|---|---|---|
| (A) |
(B) |
(C) |
(D) |
||
| (n = 5,027) | (n = 208) | (n = 1,071) | (n = 1,220) | ||
| Race, Non-Hispanic white, % | 72.3a,c | 58.8b,c | 71.6c | 80.7 | < .0001 |
| Sex, male, % | 50.2b,c | 53.6 | 55.2c | 59.4 | < .0001 |
| BMI, kg/m2 | 28.50 ± 5.87 | 28.15 ± 5.58 | 28.66 ± 6.23 | 28.51 ± 6.38 | .692 |
| Age, y | 60.99 ± 9.09a | 57.86 ± 8.86b,c | 60.66 ± 9.09 | 60.79 ± 8.55 | < .0001 |
| Smoking history, pack-years | 43.32 ± 23.57b,c | 44.29 ± 23.97c | 47.72 ± 26.72c | 52.54 ± 27.91 | < .0001 |
| Current smoking | 38.9a,b,c | 67.8b | 56.5c | 61.7 | < .0001 |
| FEV1% pred | 80.54 ± 25.85a,b,c | 73.58 ± 27.18c | 69.85 ± 27.80c | 64.80 ± 26.49 | < .0001 |
| FVC% pred | 90.68 ± 17.15b,c | 88.06 ± 19.75c | 85.05 ± 19.62 | 83.92 ± 19.02 | < .0001 |
| FEV1/FVC | 0.67 ± 0.16a,b,c | 0.63 ± 0.16c | 0.61 ± 0.17c | 0.58 ± 0.17 | < .0001 |
| 6-Min walk distance, ft | 1,417 ± 391a,b,c | 1,294 ± 395 | 1,291 ± 409 | 1,258 ± 386 | < .0001 |
| mMRC dyspnea score | 1.04 ± 1.32a,b,c | 1.76 ± 1.49c | 1.73 ± 1.48c | 2.12 ± 1.44 | < .0001 |
| GERD, % | 24.2a,b,c | 29.9 | 28.6c | 32.0 | < .0001 |
| Noct awake cough, % | 13.6a,b,c | 44.1c | 39.6c | 51.5 | < .0001 |
| Noct awake SOB, % | 12.7a,b,c | 38.4b | 28.4c | 38.4 | < .0001 |
| Allergic nasal sympt, % | 41.3a,b,c | 60.2c | 59.1c | 69.0 | < .0001 |
| Allergic ocular sympt, % | 36.3a,b,c | 54.5b | 48.0c | 54.7 | < .0001 |
| Dusty job ever, % | 41.5a,b,c | 59.2b | 50.2c | 58.5 | < .0001 |
| Fumes job ever, % | 43.3a,b,c | 57.3b,c | 49.3c | 59.4 | < .0001 |
| Exac freq, No./pt/y | 0.26 ± 0.72a,b,c | 0.63 ± 1.16c | 0.60 ± 1.16c | 0.82 ± 1.36 | < .0001 |
| History of severe exac, % | 7.9a,b,c | 18.0 | 17.8 | 20.3 | < .0001 |
| % Emphysema | 6.71 ± 9.94b,c | 6.38 ± 9.93c | 7.96 ± 10.61 | 8.86 ± 10.85 | < .0001 |
| % Gas trapping | 22.59 ± 20.04b,c | 23.27 ± 20.59c | 27.53 ± 21.97 | 29.82 ± 21.51 | < .0001 |
| Longitudinal follow-up | |||||
| Exac freq, No./pt/y | 0.30 ± 0.81b,c | 0.45 ± 0.94c | 0.64 ± 1.27c | 0.75 ± 1.24 | < .0001 |
| Exac freq ≥ 2/y, % | 3.5b,c | 4.8b,c | 10.4 | 11.6 | < .0001 |
| Severe exac freq, No./pt/y | 0.10 ± 0.39b,c | 0.14 ± 0.36c | 0.23 ± 0.68 | 0.28 ± 0.79 | < .0001 |
| Severe exac freq ≥ 1/y, % | 2.9b,c | 4.7 | 7.3 | 7.9 | < .0001 |
Figure 1.
Percent breakdown of each of the four chronic bronchitis groups. SGRQ = St. George’s Respiratory Questionnaire.
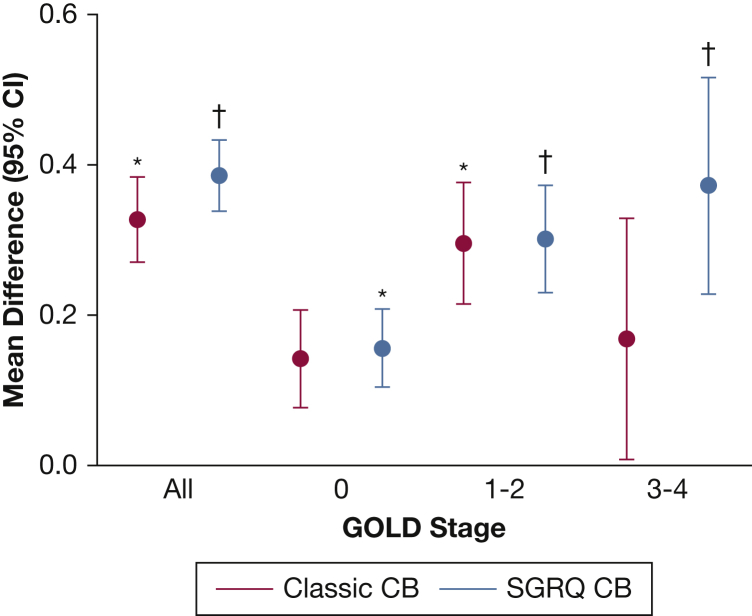
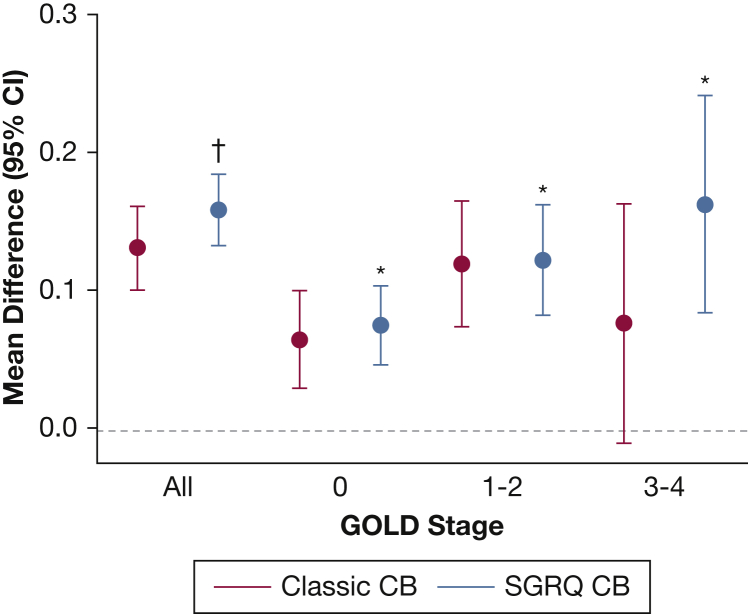
The regression coefficients for the multivariable linear regressions for exacerbation frequency and severe exacerbation frequency are shown in Tables 4 and 5. Both classic CB and SGRQ CB were independently associated with exacerbation frequency in the entire cohort (β coefficient, 0.083; SE, 0.030; P = .006; and β coefficient, 0.166; SE, 0.026; P < .0001, respectively). When divided by GOLD stages, classic CB was only independently associated with exacerbation frequency in GOLD stages 1-2, whereas SGRQ CB was associated with exacerbation frequency in all GOLD stages. Directly comparing regression coefficients, SGRQ CB was superior to classic CB in association with total exacerbation frequency in the entire cohort, as well as in GOLD 3-4 subjects, suggesting better predictive capacity (P = .017 and P = .038, respectively). SGRQ CB, but not classic CB, was independently associated with severe exacerbation frequency in all GOLD stages (β coefficient, 0.065; SE, 0.014; P < .0001; and β coefficient, 0.032; SE, 0.016; P = .054; respectively). Comparing regression coefficients, there was a trend toward a statistical difference between SGRQ CB and classic CB in the entire cohort and in GOLD 3-4 subjects (P = .077 and P = .092, respectively).
Table 4.
Multivariable Linear Regression for Exacerbation Frequency
| GOLD | Classic CB |
SGRQ CB |
SGRQ vs Classic |
||||
|---|---|---|---|---|---|---|---|
| β Coeffa | SE | P Value | β Coeffa | SE | P Value | P Value | |
| All | 0.083 | 0.030 | .006 | 0.166 | 0.026 | < .0001 | .017 |
| 0 | 0.031 | 0.035 | .366 | 0.059 | 0.029 | .039 | .391 |
| 1, 2 | 0.134 | 0.045 | .003 | 0.193 | 0.040 | < .0001 | .239 |
| 3, 4 | 0.096 | 0.095 | .312 | 0.310 | 0.088 | < .0001 | .038 |
Probability values in boldface indicate significance. Coeff = coefficient. See Table 1 legend for expansion of other abbreviations.
Age, race, sex, pack-year history, current smoking, oxygen use, modified Medical Research Council dyspnea score, % emphysema, prior exacerbation frequency, FEV1% predicted, 6-min walk distance, and BMI as covariates.
Table 5.
Multivariable Linear Regression for Severe Exacerbation Frequency
| GOLD | Classic CB |
SGRQ CB |
SGRQ vs Classic |
||||
|---|---|---|---|---|---|---|---|
| β Coeffa | SE | P Value | β Coeffa | SE | P Value | P Value | |
| All | 0.032 | 0.016 | .054 | 0.065 | 0.014 | < .0001 | .077 |
| 0 | 0.027 | 0.018 | .139 | 0.035 | 0.015 | .017 | .655 |
| 1, 2 | 0.033 | 0.026 | .199 | 0.054 | 0.023 | .018 | .520 |
| 3, 4 | 0.038 | 0.051 | .462 | 0.121 | 0.047 | .011 | .092 |
Probability values in boldface indicate significance. See Table 1 and 4 legends for expansion of abbreviations.
Age, race, sex, pack-year history, current smoking, oxygen use, modified Medical Research Council dyspnea score, % emphysema, prior exacerbation frequency, FEV1% predicted, 6-min walk distance, and BMI as covariates.
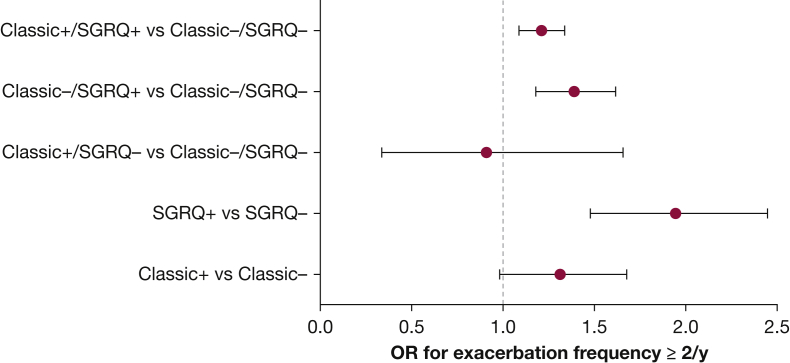
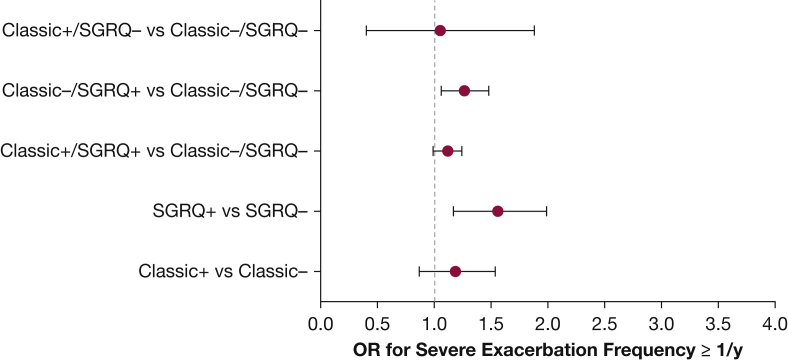
Figures 2 and 3 represent the mean differences in exacerbation frequencies and severe exacerbation frequencies, respectively, for classic CB and SGRQ CB. ORs for exacerbation frequency ≥ 2/year and severe exacerbation frequency ≥ 1/year are shown in Figures 4 and 5. SGRQ CB+ but not classic CB+ had increased ORs for exacerbation frequency ≥ 2/year in multivariable logistic regression. In addition, classic CB–/SGRQ CB+ and classic CB+/SGRQ CB+ compared with classic CB–/SGRQ CB– had increased odds of having an exacerbation frequency ≥ 2/year. Similarly, SGRQ CB+ but not classic CB+ conferred an increased risk of severe exacerbation frequency ≥ 1/year. In the four-group comparison, only the classic CB–/SGRQ CB+ group increased odds of a higher severe exacerbation frequency.
Figure 2.
Differences in exacerbation frequency across GOLD stages. Red, classic CB+ vs classic CB–; blue, SGRQ CB+ vs SGRQ CB–. Circles represent mean difference and error bars represent 95% CI. *P < .05 and †P < .0001 compared with classic CB– or SGRQ CB–, respectively, on multivariable linear regression. CB = chronic bronchitis; GOLD = Global Initiative for Chronic Obstructive Lung Disease. See Figure 1 legend for expansion of other abbreviation.
Figure 3.
Differences in severe exacerbation frequency across GOLD stages. Red, classic CB+ vs classic CB–; blue, SGRQ CB+ vs SGRQ CB–. Circles represent mean difference and error bars represent 95% CI. *P < .05 and †P < .0001 compared with classic CB– or SGRQ CB–, respectively, on multivariable linear regression. See Figure 1 and 2 legends for expansion of abbreviations.
Figure 4.
OR for exacerbation frequency ≥ 2/y. See Figure 1 legend for expansion of abbreviation.
Figure 5.
OR for severe exacerbation frequency ≥ 1/y. See Figure 1 legend for expansion of abbreviation.
Discussion
We showed in a cohort of over 7,000 current or former smokers with GOLD 0-4 disease that those with CB defined by either the SGRQ or classic definition had worse lung function, dyspnea, exercise intolerance, radiographic evidence of disease, and exacerbation histories. We again demonstrated that the SGRQ CB+ group was much larger than the classic CB+ group with similar clinical phenotypes. Most importantly, we showed that CB by both the SGRQ and classic definitions increased risk for future exacerbations, but only SGRQ CB was independently associated with severe exacerbations. To date, this is the largest extensively characterized cohort where a prospective analysis of exacerbations has been performed, with two different CB definitions directly compared against one another.
Given that there were few subjects in the classic CB+/SGRQ CB– group but many subjects in the classic CB–/SGRQ CB+ group, our analysis suggests that the SGRQ CB definition is better at identifying subjects at risk. In addition, given that the ORs for severe exacerbation frequency were not statistically different for classic CB+, and severe exacerbation frequency was similarly greater in the classic CB–/SGRQ CB+ group and the classic CB+/SGRQ CB+ group, the data suggest that the SGRQ CB definition may be at least equivalent if not better in predicting future severe exacerbations.
The prevalence of CB varies greatly among studies, ranging from a low as 2.6% in the general population to as high as 74% in small COPD cohorts.2 Much of this variation has to do with a range of definitions, including productive cough, chronic cough and expectoration, bronchial hypersecretion, and chronic sputum production.2 As a result, it has been difficult to cohesively summarize the literature regarding not only prevalence estimates but also sequelae. Therefore, it is of paramount importance to have a uniformly accepted definition to improve our understanding of this disorder.
CB affects approximately 10 million people in the United States and is associated with numerous clinical consequences.19 A prior cross-sectional analysis of the COPDGene cohort found that COPD subjects with CB had higher mMRC dyspnea scores, lower 6-minute walk distance, and worse health-related quality of life.20 Chronic mucus hypersecretion has been shown to hasten lung function decline in the Copenhagen City Heart Study, and classically defined CB also has been associated with a faster rate of decline in FEV1 in smokers without airflow obstruction.3, 21 Several studies as well have linked CB with increased all-cause and COPD-related mortality.4, 5, 7, 22
Classically defined CB has been associated with exacerbations in several studies. A cross-sectional analysis of COPDGene showed that those with classically defined CB had nearly double the number of exacerbations in the year prior compared with those without CB.20 Another cross-sectional analysis of 974 COPD subjects with GOLD 2-4 disease found that those with CB had more exacerbations, a higher percentage of patients with frequent exacerbations, and increased COPD-related and all-cause hospitalization rates.23 Classically defined CB has also been associated with respiratory exacerbations in GOLD 0 subjects.24 Similarly, the classic definition of CB was shown to be independently associated with exacerbations in the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS).25
CB has also been linked to exacerbations when defined differently. Chronic mucus hypersecretion is associated with a greater than twofold risk of COPD-related hospitalization.3 A multicenter study showed that chronic cough and phlegm had an increased exacerbation frequency.6 A large study of five Latin American cities showed that CB defined as chronic phlegm on most days for 3 months/year for ≥ 2 years was associated with more exacerbations.26
The SGRQ definition of CB has been used in several studies. In the MACRO trial, SGRQ-defined CB was determined not to be an independent predictor of response to daily azithromycin.14 We have previously shown in COPDGene that in those with GOLD 1-4 disease the SGRQ CB definition identified greater than 50% more subjects with a similar history of total and severe exacerbations than the classic CB definition.16 An analysis of smokers with and without COPD in SPIROMICS showed that the SGRQ CB definition at baseline was an independent predictor of exacerbations in the first year.25
The SGRQ assesses health-related quality of life over the prior 4 weeks. Therefore, the SGRQ CB definition can be criticized for not ascertaining chronicity of cough and phlegm adequately, as the classic definition does. However, our data show that the total and severe exacerbation frequencies by either definition were similar, and in multivariable analysis the SGRQ definition was at least similar as a predictor of severe exacerbations and a better predictor for total exacerbations. This suggests that a simple assessment of cough and phlegm in the preceding 4 weeks has greater prognostic value than ascertaining the sometimes waxing and waning symptoms over the last 2 years.
There are several limitations that are worthy of mention. First, the exacerbations were assessed by patient report and these events were not adjudicated by medical records, leaving the possibility of recall bias. There also may have been exacerbations not reported. Several studies have shown that many exacerbations noted by symptom diaries were not reported to a health-care professional.27, 28 In addition, given the discrepancy between the numbers in the classic CB+ and SGRQ CB+ groups, there may be some misclassification bias. Indeed, differences in the administration of the questionnaires (self or by a clinician) may have affected classification of subjects. Last, as exacerbations were only assessed at regularly scheduled intervals, we were unable to assess the time to first exacerbation in this LFU cohort.
Nonetheless, we have shown several advantages of the SGRQ CB definition compared with the classic one. We have shown that the SGRQ CB definition identifies 50% more subjects with GOLD 0-4 disease who have the same clinical phenotype as the classic definition; it is comparable if not better in predicting future total exacerbations; and SGRQ CB is independently associated with future severe exacerbations where classic CB was not. The SGRQ CB definition is easier to derive than the classic one, making it more clinically useful. Based on these data, a quick assessment of cough and sputum symptoms may offer better prognostic value than trying to derive the classic CB definition from multiple questions in a clinical setting. Validation of these findings in other cohorts and a prospective study comparing the two definitions would further solidify the role of the SGRQ CB definition in clinical practice.
Acknowledgments
Author contributions: V. K. conceived and designed the analysis plan, performed data analysis, and contributed significantly to the writing of the manuscript. H. Z. performed the data analysis and designed the analysis plan. E. R. acquired data used in the manuscript and contributed to the data analysis. M. K. H. contributed to the data analysis and writing of the manuscript. B. J. M. contributed to the writing of the manuscript. J. D. C., P. W. J., J. L. C., and G. J. C. contributed to the conception of the analysis plan and writing of the manuscript. E. K. S. contributed to the data analysis and the writing of the manuscript.
Financial/nonfinancial disclosures: V. K. has received personal fees from CSA Medical, AstraZeneca, Boehringer Ingelheim, and Concert Pharmaceuticals. V. K. has also received personal fees from the American Board of Internal Medicine and Gala Therapeutics. M. K. H. reports consulting for GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca. She has received research support from Novartis and Sunovion. B. J. M. reports funding from the NHLBI for the COPDGene study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovion; personal fees for DSMB from Spiration and Shire/Baxalta; CME personal fees from WebMD, National Jewish Health, the American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications Medscape, Eastern VA Medical Center, Academy for Continued Healthcare Learning, and Mt. Sinai Medical Center; royalties from Up-To-Date; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Vernoa; grants from Pearl; outside the submitted work. J. L. C. reports a grant from NHLBI directly related to this study, and grants from NIAID, NHLBI, the Department of Veterans Affairs, Department of Defense, and MedImmune Corp. Ltd, outside the scope of this study. E. K. S. received honoraria from Novartis for Continuing Medical Education Seminars and grant and travel support from GlaxoSmithKline. G. J. C. has received grants from the US NIH and the Department of Defense, consulting from AstraZeneca, Boehringer Ingelheim, Holaira, Mereo, Third Pole, PneumRx, Pulmonx, Pearl, Amirall, CSA Medical, Broncus, AVISA, Lungpacer, and GlaxoSmithKline; and contracted clinical trials from AstraZeneca, Avisa, Mereo, Boehringer Ingelheim, Broncus, GlaxoSmithKline, Lungpacer, Novartis, Pulmonx, PneumRx/BTG, and Yungjin. None declared (H. Z., E. R., J. D. C., P. W. J.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
*COPDGene Investigators Collaborators—Core Units: Administrative Center: James D. Crapo, MD (PI); Edwin K. Silverman, MD, PhD (PI); Barry J. Make, MD; Elizabeth A. Regan, MD, PhD. Genetic Analysis Center: Terri Beaty, PhD; Ferdouse Begum, PhD; Robert Busch, MD; Peter J. Castaldi, MD, MSc; Michael Cho, MD; Dawn L. DeMeo, MD, MPH; Adel R. Boueiz, MD; Marilyn G. Foreman, MD, MS; Eitan Halper-Stromberg; Nadia N. Hansel, MD, MPH; Megan E. Hardin, MD; Lystra P. Hayden, MD, MMSc; Craig P. Hersh, MD, MPH; Jacqueline Hetmanski, MS, MPH; Brian D. Hobbs, MD; John E. Hokanson, MPH, PhD; Nan Laird, PhD; Christoph Lange, PhD; Sharon M. Lutz, PhD; Merry-Lynn McDonald, PhD; Margaret M. Parker, PhD; Dandi Qiao, PhD; Elizabeth A. Regan, MD, PhD; Stephanie Santorico, PhD; Edwin K. Silverman, MD, PhD; Emily S. Wan, MD; Sungho Won. Imaging Center: Jean-Paul Charbonnier; Harvey O. Coxson, PhD; MeiLan K. Han, MD, MS; Eric A. Hoffman, PhD; Stephen Humphries, PhD; Francine L. Jacobson, MD, MPH; Philip F. Judy, PhD; Ella A. Kazerooni, MD; Alex Kluiber; David A. Lynch, MB; John D. Newell, Jr., MD; Elizabeth A. Regan, MD, PhD; James C. Ross, PhD; Raul San Jose Estepar, PhD; Jered Sieren; Berend C. Stoel, PhD; Juerg Tschirren, PhD; Edwin Van Beek, MD, PhD; Bram van Ginneken, PhD; Eva van Rikxoort, PhD; George Washko, MD; Carla G. Wilson, MS. PFT QA Center, Salt Lake City, UT: Robert Jensen, PhD. Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, PhD; Jim Crooks, PhD; Camille Moore, PhD; Matt Strand, PhD; Carla G. Wilson, MS. Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, MPH, PhD; John Hughes, PhD; Gregory Kinney, MPH, PhD; Sharon M. Lutz, PhD; Katherine Pratte, MSPH; Kendra A. Young, PhD.
Clinical Centers: Ann Arbor VA, Ann Arbor, MI: Jeffrey L. Curtis, MD; Carlos H. Martinez, MD, MPH; Perry G. Pernicano, MD. Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS; Philip Alapat, MD; Mustafa Atik, MD; Venkata Bandi, MD; Aladin Boriek, PhD; Kalpatha Guntupalli, MD; Elizabeth Guy, MD; Arun Nachiappan, MD; Amit Parulekar, MD. Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, MD, MPH; Craig Hersh, MD, MPH; Francine L. Jacobson, MD, MPH; George Washko, MD. Columbia University, New York, NY: R. Graham Barr, MD, DrPH; John Austin, MD; Belinda D’Souza, MD; Gregory D. N. Pearson, MD; Anna Rozenshtein, MD, MPH, FACR; Byron Thomashow, MD. Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD; H. Page McAdams, MD; Lacey Washington, MD. HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, MD, MPH; Joseph Tashjian, MD. Johns Hopkins University, Baltimore, MD: Robert Wise, MD; Robert Brown, MD; Nadia N. Hansel, MD, MPH; Karen Horton, MD; Allison Lambert, MD, MHS; Nirupama Putcha, MD, MHS. Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, PhD, MD; Alessandra Adami, PhD; Matthew Budoff, MD; Hans Fischer, MD; Janos Porszasz, MD, PhD; Harry Rossiter, PhD; William Stringer, MD. Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD, PhD; Charlie Lan, DO. Minneapolis VA, Minneapolis, MN: Christine Wendt, MD; Brian Bell, MD. Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, MD, MS; Eugene Berkowitz, MD, PhD; Eric L. Flenaugh, MD; Gloria Westney, MD, MS. National Jewish Health, Denver, CO: Russell Bowler, MD, PhD; David A. Lynch, MB. Reliant Medical Group, Worcester, MA: Richard Rosiello, MD; David Pace, MD. Temple University, Philadelphia, PA: Gerard Criner, MD; David Ciccolella, MD; Francis Cordova, MD; Chandra Dass, MD; Gilbert D’Alonzo, DO; Parag Desai, MD; Michael Jacobs, PharmD; Steven Kelsen, MD, PhD; Victor Kim, MD; A. James Mamary, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; Kartik Shenoy, MD; Robert M. Steiner, MD; Maria Elena Vega-Sanchez, MD. University of Alabama, Birmingham, AL: Mark Dransfield, MD; William Bailey, MD; Surya Bhatt, MD; Anand Iyer, MD; Hrudaya Nath, MD; Gabriela Oates, PhD; Sushil Sonavane, MD; J. Michael Wells, MD. University of California, San Diego, CA: Joe Ramsdell, MD; Paul Friedman, MD; Xavier Soler, MD, PhD; Andrew Yen, MD. University of Iowa, Iowa City, IA: Alejandro P. Comellas, MD; John Newell, Jr., MD; Brad Thompson, MD. University of Michigan, Ann Arbor, MI: MeiLan K. Han, MD, MS; Ella Kazerooni, MD; Carlos H. Martinez, MD, MPH. University of Minnesota, Minneapolis, MN: Joanne Billings, MD; Abbie Begnaud, MD; Tadashi Allen, MD. University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD; Jessica Bon, MD; Divay Chandra, MD, MSc; Carl Fuhrman, MD; Joel Weissfeld, MD, MPH. University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD; Sandra Adams, MD; Diego Maselli-Caceres, MD; Mario E. Ruiz, MD.
Footnotes
FUNDING/SUPPORT: The project described was supported by the National Heart, Lung, and Blood Institute [Awards U01 HL089897 and U01 HL089856]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. COPD Foundation Funding: The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion.
Contributor Information
Victor Kim, Email: victor.kim@tuhs.temple.edu.
COPDGene Investigators:
James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri Beaty, Ferdouse Begum, Robert Busch, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Nadia N. Hansel, Megan E. Hardin, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Stephanie Santorico, Edwin K. Silverman, Emily S. Wan, Sungho Won, Jean-Paul Charbonnier, Harvey O. Coxson, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Jered Sieren, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, Carla G. Wilson, John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, Kendra A. Young, Jeffrey L. Curtis, Carlos H. Martinez, Perry G. Pernicano, Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Gregory D.N. Pearson, Anna Rozenshtein, Byron Thomashow, Neil MacIntyre, Jr., H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Marilyn G. Foreman, Eugene Berkowitz, Eric L. Flenaugh, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, Gabriela Oates, Sushil Sonavane, J. Michael Wells, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro P. Comellas, John Newell, Jr., Brad Thompson, MeiLan K. Han, Ella Kazerooni, Carlos H. Martinez, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz
Supplementary Data
References
- 1.US Burden of Disease Collaborators. Mokdad A.H., Ballestros K., Echko M. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. doi: 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim V., Criner G.J. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med. 2015;21(2):133–141. doi: 10.1097/MCP.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J., Prescott E., Lange P., Copenhagen City Heart Study Group Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153(5):1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 4.Pelkonen M., Notkola I.L., Nissinen A., Tukiainen H., Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130(4):1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 5.Guerra S., Sherrill D.L., Venker C., Ceccato C.M., Halonen M., Martinez F.D. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64(10):894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgel P.R., Nesme-Meyer P., Chanez P. Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 7.Pelkonen M.K., Notkola I.K., Laatikainen T.K., Jousilahti P. Chronic bronchitis in relation to hospitalization and mortality over three decades. Respir Med. 2017;123:87–93. doi: 10.1016/j.rmed.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Lange P., Nyboe J., Appleyard M., Jensen G., Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax. 1990;45(8):579–585. doi: 10.1136/thx.45.8.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallasaho P., Lundback B., Laspa S.L. Increasing prevalence of asthma but not of chronic bronchitis in Finland: report from the FinEsS-Helsinki Study. Respir Med. 1999;93:798–809. doi: 10.1016/s0954-6111(99)90265-2. [DOI] [PubMed] [Google Scholar]
- 10.Sobradillo V., Miravitlles M., Jimenez C.A. Epidemiological study of chronic obstructive pulmonary disease in Spain (IBERPOC): prevalence of chronic respiratory symptoms and airflow limitation. Arch Bronconeumol. 1999;35(4):159–166. doi: 10.1016/s0300-2896(15)30272-6. [DOI] [PubMed] [Google Scholar]
- 11.Janson C., Chinn S., Jarvis D., Burney P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J. 2001;18(4):647–654. doi: 10.1183/09031936.01.00098701. [DOI] [PubMed] [Google Scholar]
- 12.de Marco R., Accordini S., Cerveri I. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175(1):32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 13.Ferris B.G. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Part 2):1–120. [PubMed] [Google Scholar]
- 14.Han M.K., Tayob N., Murray S. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503–1508. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim V., Sternberg A.L., Washko G., National Emphysema Treatment Trial Research Group Severe chronic bronchitis in advanced emphysema increases mortality and hospitalizations. COPD. 2013;10(6):667–678. doi: 10.3109/15412555.2013.827166. [DOI] [PubMed] [Google Scholar]
- 16.Kim V., Crapo J., Zhao H. COPDGene Investigators. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan E.A., Hokanson J.E., Murphy J.R. Genetic Epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart J.I., Moyle S., Criner G.J. COPDGene Investigators. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Lung Association Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality. March 2013. https://www.lung.org/assets/documents/research/copd-trend-report.pdf
- 20.Kim V., Han M.K., Vance G.B. COPDGene Investigators. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez C.H., Putcha N., Labaki W. In subjects with non-obstructive chronic bronchitis, lung function decline and clinical outcomes vary by sex, race and smoking status: a longitudinal analysis of the COPDGene cohort [abstract] Am J Respir Crit Care Med. 2017;195:A2720. [Google Scholar]
- 22.Prescott E., Lange P., Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8(8):1333–1338. doi: 10.1183/09031936.95.08081333. [DOI] [PubMed] [Google Scholar]
- 23.Corhay J.L., Vincken W., Schlesser M., Bossuyt P., Imschoot J. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract. 2013;67(12):1294–1301. doi: 10.1111/ijcp.12248. [DOI] [PubMed] [Google Scholar]
- 24.Martinez C.H., Kim V., Chen Y. COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108:491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim V., O’Neal W.K., Ford A.A. The Saint George’s Respiratory Questionnaire definition of chronic bronchitis identifies more subjects at risk for exacerbations than the classic definition and is stable over time: a prospective analysis of the SPIROMICS cohort [abstract] Am J Respir Crit Care Med. 2017;195:A2890. [Google Scholar]
- 26.de Oca M.M., Halbert R.J., Lopez M.V. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 27.Langsetmo L., Platt R.W., Ernst P., Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 28.Vijayasaratha K., Stockley R.A. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest. 2008;133(1):34–41. doi: 10.1378/chest.07-1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.