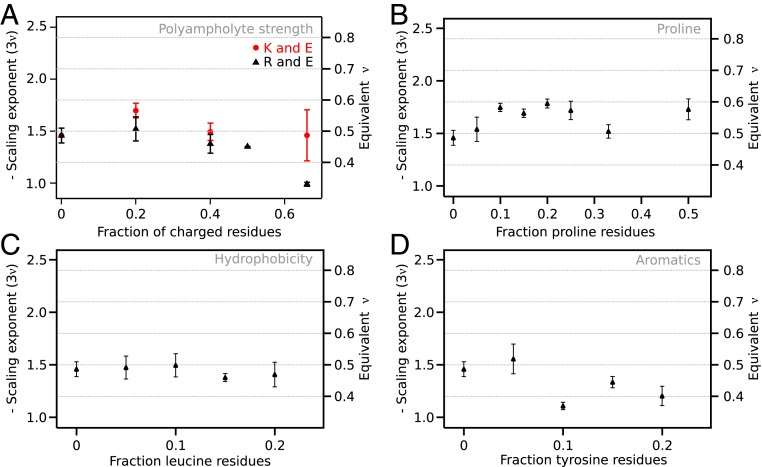
Fig. 4.
The effect of polyampholyte strength and proline, leucine, and tyrosine residues on scaling exponents. (A) Scaling exponents from neutral polyampholyte linkers with an equal proportion of glutamate residues and either lysine or arginine. The scaling exponents show a strong compaction to a globular chain for polyampholytes containing arginine only. (B) Chain expansion shows a complex dependence on proline content. At low fractions of proline residues, proline residues lead to linker expansion, which may be reversed at high proline fractions. (C) Hydrophobicity was increased by introduction of leucine residues, but led to practically no change in chain compaction. (D) Tyrosine residues led to strong compaction, likely due to π-interactions. Error bars are SE estimated from the fit.