Abstract
In patients with atrial fibrillation (AF), the prevalence of moderate-to-severe sleep-disordered breathing (SDB) ranges between 21% and 72% and observational studies have demonstrated that SDB reduces the efficacy of rhythm control strategies, while treatment with continuous positive airway pressure lowers the rate of AF recurrence. Currently, the number of apneas and hypopneas per hour (apnea-hypopnea-index, AHI) determined during a single overnight sleep study is clinically used to assess the severity of SDB. However, recent studies suggest that SDB-severity in an individual patient is not stable over time but exhibits a considerable night-to-night variability which cannot be detected by only one overnight sleep assessment. Nightly SDB-severity assessment rather than the single-night diagnosis by one overnight sleep study may better reflect the exposure to SDB-related factors and yield a superior metric to determine SDB-severity in the management of AF.
In this review we discuss mechanisms of night-to-night SDB variability, arrhythmogenic consequences of night-to-night SDB variability, strategies for longitudinal assessment of nightly SDB-severity and clinical implications for screening and management of SDB in AF patients.
1. Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with significant morbidity, increased risk of stroke, reduced quality of life, and increased mortality [1]. Concomitant risk factors such as hypertension, obesity, metabolic syndrome and ageing lead to structural remodeling processes in the atria which contribute to the progressive nature of AF and the reduced efficacy of standard antiarrhythmic pharmacological and catheter-based rhythm control strategies [2], [3], [4].
Another emerging risk factor for AF is sleep-disordered breathing (SDB). In patients with AF, the prevalence of categorically diagnosed moderate-to-severe SDB ranges between 21% and 72%, and this variation in reported prevalence is due to the nature of the cohorts studied as well as the differing scoring criteria and thresholds used for defining SDB [5], [6]. Observational studies have demonstrated that SDB reduces the efficacy of catheter- and pharmacological- based rhythm control strategies, while treatment with continuous positive airway pressure (CPAP) lowers the rate of AF recurrence after electrical cardioversion and improves catheter ablation outcomes [7], [8], [9], [10]. Based on these data, international AF guidelines recommend screening for signs and symptoms of sleep apnea and CPAP treatment when evaluating patients for rhythm control to reduce AF-recurrence and improve AF treatment results [11].
In sleep medicine, the number of apneas and hypopneas per hour (apnea-hypopnea-index, AHI) determined during a single overnight sleep study is clinically used to assess the severity of SDB [12]. Patients are categorized into having SDB when the AHI is ≥5/h. The severity of SDB is further graded, albeit quite arbitrarily, into mild (AHI 5–15), moderate (AHI 15–30) and severe (AHI ≥ 30) [13]. However, recent studies suggest that SDB-severity in an individual patient is not stable over time but exhibits a considerable night-to-night variability which cannot be detected by only one overnight sleep assessment [14], [15], [16], [17], [18], [19]. Nights with more respiratory events (higher AHI) may be related to a higher AF risk compared to nights with less respiratory events (lower AHI). Therefore, nightly SDB-severity assessment rather than the single-night diagnosis by one overnight sleep study may better reflect the exposure to SDB-related factors and yield a superior metric to determine SDB-severity in the management of AF.
In this review we discuss mechanisms of night-to-night SDB variability, arrhythmogenic consequences of night-to-night SDB variability, strategies for longitudinal assessment of nightly SDB-severity and clinical implications for screening and management of SDB in AF patients.
2. Mechanisms of night-to-night SDB variability in AF
Traditionally, assessment of SDB-severity is based on the result of one overnight sleep study. However, SDB patients with and without concomitant cardiovascular disease show considerable intra-individual night-to-night variability in AHI [14], [15], [16], [17], [18], [19]. Importantly, the intra-individual short-term night-to-night variability is higher in patients with mild SDB than in those with moderate to severe SDB.
2.1. General mechanisms
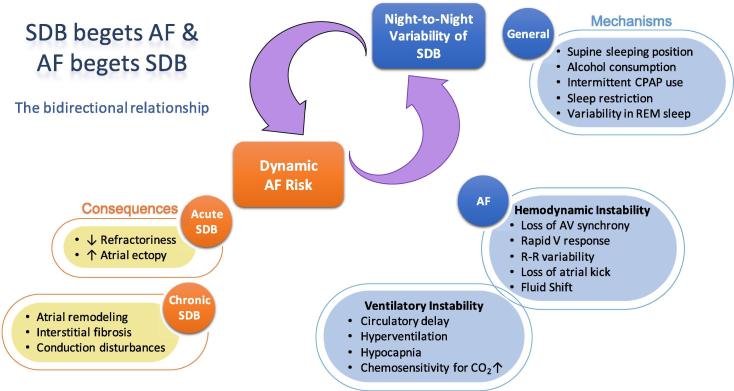
In the individual patient, between-night differences in SDB-severity may depend on positional factors such as time spent sleeping in the supine position. In positional obstructive sleep apnea, not just the position of the trunk, but also the degree of head rotation and flexion/extension can impact on the severity of SDB [20]; while elevation of the upper body can improve SDB-severity [21], [22]. SDB episodes predominantly occur during REM sleep in most patients. Sleep restriction with early termination of sleep (and thus less REM-sleep), sleep disturbances in general (first-night-effect in a sleep lab, jetlag) or intermittent non-use of antidepressants (with REM-rebound) may result in a variation in the amount of REM-sleep and may impact nightly SDB-severity. Further, intermittent CPAP use as well as alcohol consumption or use of hypnotics before bedtime may change arousability and contribute to night-to-night variability in SDB-severity (Fig. 1).
Fig. 1.
The hypothesized bidirectional relationship between sleep-disordered breathing (SDB) and atrial fibrillation (AF): SDB begets AF and AF begets SDB. Arrhythmogenic consequences of night-to-night variability in SDB severity (in orange) and general and AF-related mechanisms of night-to-night variability in SDB (in blue).
2.2. Mechanisms specific for patients with AF
During AF, loss of AV synchrony, a rapid ventricular response, a high beat-to-beat variability and the absence of atrial contraction (atrial kick) impair ventricular hemodynamics. Subsequently, the altered hemodynamics, which in turn can be variable on a nightly basis, can contribute to the dynamic SDB-severity. Emerging evidence points towards a crucial involvement of cardiovascular hemodynamics and nocturnal fluid shifts (due to variable fluid intake, use of diuretics and physical activity vs. sedentary behavior) in the genesis of particularly central but also obstructive respiratory events in AF patients (“AF begets SDB”) [23]. Non-anatomical factors such as arousability, loop gain and upper airway muscular responsiveness during sleep have been implicated in up to 56% of patients with obstructive sleep apnea and may contribute to night-to-night variability [24]. Increased sensitivity of peripheral and central chemoreceptors, pulmonary congestion and prolonged circulation time all contribute to the dysregulation of respiratory control and are associated with more prolonged events [6], [23], [25]. Accordingly, in patients with persistent AF, rhythm control by electrical cardioversion did not have an impact on the absolute AHI but reduced nocturnal central respiratory events and “unmasked” obstructive sleep apnea [26].
3. Arrhythmogenic consequences of night-to-night SDB variability in AF
Night-to-night variability in SDB-severity might result in a variable and dynamic exposure to SDB-related conditions which may impact the timing and extent of cardiovascular responses such as the onset of AF (“SDB begets AF”) (Fig. 1) [27]. Severe long-term SDB has been shown to be associated with structural remodeling in the atria in humans and animal models [28], [29], [30]. Mechanistically, obstructive respiratory events may cause structural remodeling and myocardial damage through repetitive mechanical atrial distension and atrial wall stretch as well as frequent episodes of oxyhemoglobin desaturation-resaturation. On top of the chronic structural alterations in the atria, transient apnea-associated autonomically-mediated shortening in action potential duration, increases in atrial ectopy and transient changes in conduction velocity create a dynamic arrhythmogenic substrate in the atria for enhanced AF susceptibility [31], [32], [33], [34], [35], [36]. This transient increase in atrial arrhythmogenesis may depend on nightly variability in SDB-severity and contribute to the temporal relationship between increased occurrence of arrhythmias during or after sleep apnea episodes [37], [38], [39], [40].
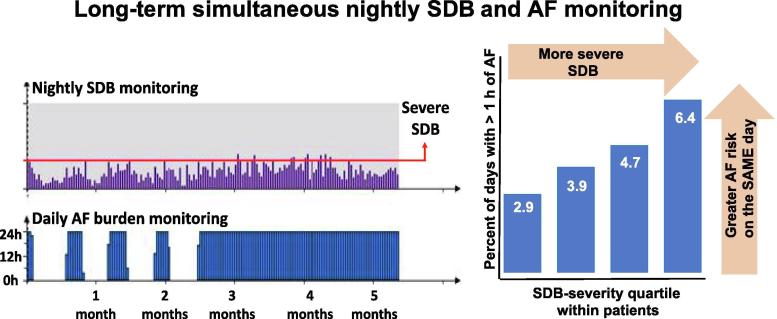
Longitudinally assessed SDB-severity (high SDB-burden), rather than the result of one overnight sleep study, may better reflect the exposure to SDB-related AF risk. In patients with implanted pacemakers, using simultaneous long-term night-by-night SDB and AF monitoring, we characterized night-to-night variability in SDB-severity and examined the relationship between SDB and AF with each patient acting as their own control (The VARIability in severity Of Sleep Apnea and nightly dynamic Atrial Fibrillation risk (VARI-OSA-AF) study) [40], [41]. In individual patients, the nights with the highest SDB-severity conferred a more than 2.3-fold increased risk of having at least one hour of AF the same day compared to nights with the lowest SDB-severity (Fig. 2) [40]. This suggests that increased cardiac stress induced by even a single night with more severe SDB can establish conditions predisposing to AF. Importantly, the relationship between nights with more severe SDB and increased AF-risk held for all individuals irrespective of the clinical diagnosis of SDB and mean AHI [40]. This suggests that the relative SDB-severity can trigger AF episodes and this is irrespective of underlying SDB-severity category.
Fig. 2.
An example of long-term simultaneous nightly sleep-disordered breathing (SDB) and atrial fibrillation (AF) burden monitoring (left). Note the high night-to-night variability and AF episodes. Increasing daily AF risk dependent goes hand in hand with nightly SDB severity (right).
4. Assessment of nightly SDB-severity and long-term SDB-pattern
Given the described dynamic relationship between nightly SDB-severity and AF risk, there exists a need for a metric that better captures the SDB ‘burden’ than a single overnight sleep study. Therefore, to assess nightly SDB-severity and long-term SDB-pattern (i.e. night-to-night variability in SDB), continuous long-term SDB monitoring (or monitoring of an appropriate surrogate parameter) is required. This is particularly relevant for patients in whom a high clinical suspicion of significant SDB exists but who, on a single ‘snapshot’ sleep study, do not exhibit the number of apneic or hypopneic events per hour required for a diagnosis of moderate-to-severe SDB, given the management implications associated with this category. A single sleep study or possibly even several repeated sleep studies may not be able to capture the proportion of nights spent with severe SDB and long-term SDB-pattern
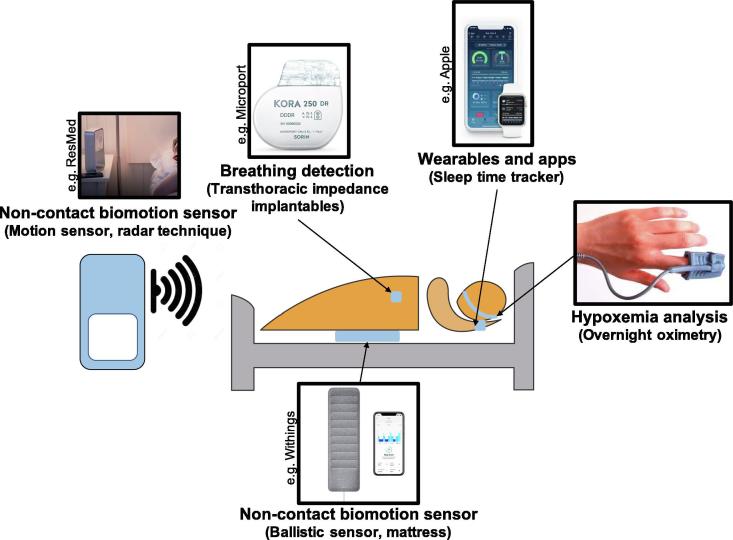
In that regard, technologies implemented in implanted pacemaker devices that derive a surrogate marker of SDB, based on transthoracic impedance changes, are able to track fluctuations in tidal volume occurring during SDB events (Fig. 3) [42], [43], [44], [45]. The available algorithms have been validated against the AHI measured in polysomnography with variable sensitivity and specificity to detect severe SDB [42], [45]. Sleep apnea monitoring by pacemakers showed a significant night-to-night variability in SDB-severity [44] and pacemaker-detected SDB has been shown to predict new onset AF in a larger cohort [45]. Potentially, comparable technologies based on transthoracic impedance changes or accelerometer measurements could also be implemented in other implantable diagnostic devices such as loop recorders.
Fig. 3.
Different approaches to assess nightly sleep-disordered breathing (SDB) severity: Non-contact biomotion sensors (e.g. radar technique, ballistic sensors, etc.); breathing detection (e.g. transthoracic impedance changes recorded by implantable devices, etc); wearables and apps; hypoxia analysis (e.g. by overnight oximetry, etc).
Non-contact biomotion sensors with actimetry, ballistic sensors or Doppler technology with radar frequencies can remotely monitor breathing during sleep (Fig. 3) [42], [43], [44]. A non‐contact biomotion radar sensor (SleepMinder™; ResMed) has been previously validated against AHI measured by polysomnography to monitor breathing during sleep remotely over periods of months to years [46], [47], [48]. Additionally, sleep tracking mats can monitor breathing disturbances during the night, and give users educational content about the signs of sleep apnea via a mobile app (Withings™; Health Mate app).
Wearable devices with inbuilt pulse oximeters are now also becoming commercially available raising the prospect of routinely measuring the night-to-night variability of SDB-severity by means of the oxygen desaturation index or determining hypoxic burden more broadly by newer validated algorithms (Fig. 3) [49], [50]. Additionally, computer analysis software of some long-term Holter systems can detect SDB by impedance changes of the surface ECG, although validation studies for long-term SDB assessment are not yet available.
Further, smartphone applications may be helpful for the purposes of large-scale, low-cost and long-term sleep monitoring (Fig. 3) [51]. Multiple applications have shown good capability in detection of sleep-wake cycles, but the reliability of smartwatch-based assessment of healthy and disturbed sleep and SDB-severity remains a key issue [52], [53]. Most of the available smartphone applications are not validated against the gold standard polysomnography and it remains unclear how monitoring could be organized in practice. However, apps can be helpful in applying dedicated in-app sleep coaching programs that can help to reduce fatigue, improve health, and support weight management efforts by leading towards a more balanced sleep schedule and managing sleep temperature, wake-up time etc.
5. Clinical implications
5.1. SDB screening
Given the high night-to-night variability, a single overnight sleep study may not be representative of true SDB-severity in a given individual. Particularly, in patients with mild-to-moderate SDB where the AHI is close to the somewhat ‘arbitrary’ cut-off values, misclassification of SDB-status may occur not infrequently. In patients with a high clinical suspicion of SDB, especially in the setting of treatment-resistant hypertension or AF recurrence despite initial success with rhythm control strategies, data from novel methods of SDB monitoring may prove to be helpful or repeat sleep studies should be considered.
5.2. SDB treatment adherence monitoring
Many patients use CPAP intermittently which results in an artificial night-to-night variability in SDB-severity. Long-term SDB monitoring could help to document therapeutic efficacy and compliance with CPAP and determine the biological response with respect to the reduction of nightly SDB-severity and AF-burden with treatment. Additionally, remote monitoring data of different CPAP machines could be made available to treating physicians (not just sleep specialists) to help assess and guide CPAP adherence. Although the appropriate AHI threshold at which to commence treatment remains unclear, a more effective reduction in nightly SDB-severity should result in a better reduction in SDB-related AF risk.
5.3. One-to-one comparisons of the SDB events and rhythm monitoring
Simultaneous SDB and AF monitoring may offer opportunities for a better understanding of how exactly SDB translates into higher AF risk. Potentially, the interaction between SDB variability and AF occurrence may help to distinguish patients where worsening in SDB-severity precedes AF episodes (“SDB begets AF”) from patients where AF episodes precedes changes in SDB-severity (“AF begets SDB“).
5.4. Clinical pathway and coordination of care
Assessment and management of SDB by continuous long-term combined AF and SDB-monitoring with a whole range of new technologies and disciplines may significantly alter how we practice SDB management in AF patients. The incorporation of these tools and data into the clinical work flow, as well as the empowering of patients through education regarding the rationale, tools (e.g. CPAP) and goals of their individualized SDB and AF treatment plans is crucial for treatment success [54]. A structured follow-up where assessment of AF burden, nightly SBD-severity, adherence and self-management of patients, as well as detection of side- and adverse effects of SDB treatment can be facilitated through a multidisciplinary approach with structured organization of care. Additionally, the utilization of monitoring tools allows informed patient-tailored management decisions. Long-term monitoring of SDB together with mobile health applications facilitates interactive feedback between patients and clinicians with respect to SDB disease progression, adherence to therapy or changes in SDB-severity upon interventions.
Additionally, SDB response to treatment or risk factor management interventions such as weight loss, exercise interventions, or behavioral interventions (sleep hygiene, alcohol abstinence, positional interventions etc.) could also be monitored by long-term sleep apnea recordings for more tailored goal directed management [55], [56], [57].
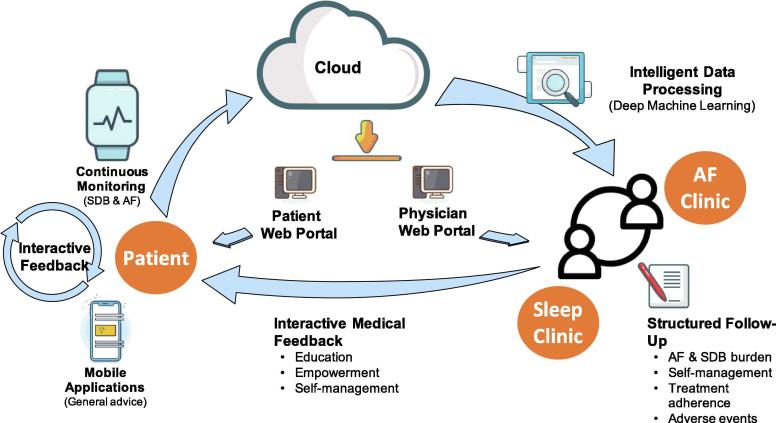
The basic principle of long-term SDB monitoring and a mobile SDB management system is shown in Fig. 4 and consists of the following modules: The patient end, which consists of a smartphone equipped with a special SDB app connected wirelessly to a long-term SDB monitoring device. The app provides general education and advice dependent on the measured SDB-severity. The acquired SDB data are transmitted and stored in a remote portal hosted in a health clinic or hospital. This portal is supported with intelligent data analytic tools (artificial intelligence, deep machine learning algorithms) that can process the data. These can be accessed and viewed by the patient and physician via two separate web portals, each designed and developed for separate access. Apps or home monitoring in the post treatment follow-up at home or as an adjunct to the sleep diary in the clinical setting may also improve treatment adherence compared with standard care by proactive management strategies [58]. Remote telemonitoring of SDB patients in terms of CPAP adherence, following SDB disease progression, AF burden and daily physical activity may be an important component of a comprehensive health monitoring and lifestyle coaching program [59].
Fig. 4.
Long-term sleep-disordered breathing (SDB) monitoring: Basic principles of a possible clinical pathway to implement long-term SDB monitoring in an atrial fibrillation (AF) clinic. For more details, see text.
6. Conclusions
Nightly SDB-severity, determined by long-term SDB monitoring, rather than the single-night diagnosis by one overnight sleep study, may better reflect SDB-associated AF risk. The ability to perform long-term monitoring may change how we manage SDB in AF patients. Future observational “real world” studies and prospective and randomized intervention studies are warranted to: (I) determine the best technology, feasibility, accuracy and cost-effectiveness of long-term SDB monitoring in the management SDB (II) affirm that treatment of “intermittent/paroxysmal SDB” leads to improved AF outcomes and (III) determine how long-term monitoring of SDB can be implemented as a component of structured risk factor management programs.
Conflict of interest
The authors declare that there is a no conflict of interest.
References
- 1.Chang T.Y., Liao J.N., Chao T.F., Vicera J.J., Lin C.Y., Tuan T.C., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Chung F.P., Chen S.A. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayinde H., Schweizer M.L., Crabb V., Ayinde A., Abugroun A., Hopson J. Age modifies the risk of atrial fibrillation among athletes: a systematic literature review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2018;18:25–29. doi: 10.1016/j.ijcha.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: imminent impulses are emerging. Int. J. Cardiol. Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelsgaard C.S., Pedersen K.B., Riber L.P., Pallesen P.A., Brandes A. The long-term efficacy of concomitant maze IV surgery in patients with atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2018;19:20–26. doi: 10.1016/j.ijcha.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linz D., McEvoy R.D., Cowie M.R., Somers V.K., Nattel S., Lévy P., Kalman J.M., Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 6.Linz D., Woehrle H., Bitter T., Fox H., Cowie M.R., Böhm M., Oldenburg O. The importance of sleep-disordered breathing in cardiovascular disease. Clin. Res. Cardiol. 2015;104:705–718. doi: 10.1007/s00392-015-0859-7. [DOI] [PubMed] [Google Scholar]
- 7.Ng C.Y., Liu T., Shehata M., Stevens S., Chugh S.S., Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011;108:47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Wang Z.W., Li J., Ge X., Guo L.Z., Wang Y., Guo W.H., Jiang C.X., Ma C.S. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. 2014;16:1309–1314. doi: 10.1093/europace/euu066. [DOI] [PubMed] [Google Scholar]
- 9.Shukla A., Aizer A., Holmes D., Fowler S., Park D.S., Scott B., Bernstein N., Chinitz L. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. J. Am. Coll. Cardiol.: Clin. Electrophysiol. 2015;1:41–51. doi: 10.1016/j.jacep.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi W.T., Nasir U.B., Alqalyoobi S., O'Neal W.T., Mawri S., Sabbagh S., Soliman E.Z., Al-Mallah M.H. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am. J. Cardiol. 2015;116:1767–1773. doi: 10.1016/j.amjcard.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 11.P. Kirchhof, S. Benussi, D. Kotecha, A. Ahlsson, D. Atar, B. Casadei, M. Castella, H.C. Diener, H. Heidbuchel, J. Hendriks, G. Hindricks, A.S. Manolis, J. Oldgren, B.A. Popescu, U. Schotten, B. Van Putte, P. Vardas, ESC Scientific Document Group, 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS, Eur. Heart J. 37 (2016) 2893–2962.
- 12.Linz D., Baumert M., Catcheside P., Floras J., Sanders P., Lévy P., Cowie M.R., Doug McEvoy R. Assessment and interpretation of sleep disordered breathing severity in cardiology: clinical implications and perspectives. Int. J. Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 13.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K., Marcus C.L., Mehra R., Parthasarathy S., Quan S.F., Redline S., Strohl K.P., Davidson Ward S.L., Tangredi M.M. American academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J. Clin. Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stöberl A.S., Schwarz E.I., Haile S.R., Turnbull C.D., Rossi V.A., Stradling J.R., Kohler M. Night-to-night variability of obstructive sleep apnea. J. Sleep Res. 2017;26:782–788. doi: 10.1111/jsr.12558. [DOI] [PubMed] [Google Scholar]
- 15.Prasad B., Usmani S., Steffen A.D., Van Dongen H.P., Pack F.M., Strakovsky I., Staley B., Dinges D., Maislin G., Pack A.I., Weaver T.E. Short-term variability in apnea-hypopnea index during extended home portable monitoring. J. Clin. Sleep Med. 2016;12:855–863. doi: 10.5664/jcsm.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmadi N., Shapiro G.K., Chung S.A., Shapiro C.M. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13:221–226. doi: 10.1007/s11325-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 17.Maestri R., La Rovere M.T., Robbi E., Pinna G.D. Night-to-night repeatability of measurements of nocturnal breathing disorders in clinically stable chronic heart failure patients. Sleep Breath. 2011;15:673–678. doi: 10.1007/s11325-010-0418-4. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg O., Lamp B., Freivogel K., Bitter T., Langer C., Horstkotte D. Low night-to-night variability of sleep disordered breathing in patients with stable congestive heart failure. Clin. Res. Cardiol. 2008;97:836–842. doi: 10.1007/s00392-008-0695-0. [DOI] [PubMed] [Google Scholar]
- 19.Vazir A., Hastings P.C., Papaioannou I., Poole-Wilson P.A., Cowie M.R., Morrell M.J., Simonds A.K. Variation in severity and type of sleep-disordered breathing throughout 4 nights in patients with heart failure. Respir. Med. 2008;102:831–839. doi: 10.1016/j.rmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Zhu K., Bradley T.D., Patel M., Alshaer H. Influence of head position on obstructive sleep apnea severity. Sleep Breath. 2017;21:821–828. doi: 10.1007/s11325-017-1525-2. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy R.D., Sharp D.J., Thornton A.T. The effects of posture on obstructive sleep apnea. Am. Rev. Respir. Dis. 1986;133:662–666. doi: 10.1164/arrd.1986.133.4.662. [DOI] [PubMed] [Google Scholar]
- 22.Neill A.M., Angus S.M., Sajkov D., McEvoy R.D. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 23.Linz D., Fox H., Bitter T., Spießhöfer J., Schöbel C., Skobel E., Türoff A., Böhm M., Cowie M.R., Arzt M., Oldenburg O. Impact of SERVE-HF on management of sleep disordered breathing in heart failure: a call for further studies. Clin. Res. Cardiol. 2016;105:563–570. doi: 10.1007/s00392-016-0970-4. [DOI] [PubMed] [Google Scholar]
- 24.Eckert D.J., White D.P., Jordan A.S., Malhotra A., Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Light M., Orr J.E., Malhotra A., Owens R.L. Continuous positive airway pressure device detects atrial fibrillation induced central sleep apnoea. Lancet. 2018;392:160. doi: 10.1016/S0140-6736(18)31381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox H., Bitter T., Horstkotte D., Oldenburg O. Cardioversion of atrial fibrillation or atrial flutter into sinus rhythm reduces nocturnal central respiratory events and unmasks obstructive sleep apnoea. Clin. Res. Cardiol. 2016;105:451–459. doi: 10.1007/s00392-015-0940-2. [DOI] [PubMed] [Google Scholar]
- 27.Linz D., Hohl M., Ukena C., Mahfoud F., Wirth K., Neuberger H.R., Böhm M. Obstructive respiratory events increase premature atrial contractions after cardioversion. Eur. Respir. J. 2015;45:1332–1340. doi: 10.1183/09031936.00175714. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki Y.K., Kato T., Xiong F., Shi Y.F., Naud P., Maguy A., Mizuno K., Tardif J.C., Comtois P., Nattel S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J. Am. Coll. Cardiol. 2014;64:2013–2023. doi: 10.1016/j.jacc.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 29.Anter E., Di Biase L., Contreras-Valdes F.M., Gianni C., Mohanty S., Tschabrunn C.M., Viles-Gonzalez J.F., Leshem E., Buxton A.E., Kulbak G., Halaby R.N., Zimetbaum P.J., Waks J.W., Thomas R.J., Natale A., Josephson M.E. Circ. Arrhythm. Electrophysiol. 2017:10. doi: 10.1161/CIRCEP.117.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitri H., Ng M., Brooks A.G., Kuklik P., Stiles M.K., Lau D.H., Antic N., Thornton A., Saint D.A., McEvoy D., Antic R., Kalman J.M., Sanders P. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson I.H., Roberts-Thomson K.C., Kistler P.M., Edwards G.A., Spence S., Sanders P., Kalman J.M. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm. 2010;7:1263–1270. doi: 10.1016/j.hrthm.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki Y.K., Shi Y., Benito B., Gillis M.A., Mizuno K., Tardif J.C., Nattel S. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm. 2012;9(1409–1416) doi: 10.1016/j.hrthm.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Linz D., Hohl M., Nickel A., Mahfoud F., Wagner M., Ewen S., Schotten U., Maack C., Wirth K., Böhm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 34.Linz D., Schotten U., Neuberger H.R., Böhm M., Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8:1436–1443. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 35.Ghias M., Scherlag B.J., Lu Z., Niu G., Moers A., Jackman W.M., Lazzara R., Po S.S. The role of ganglionated plexi in apnea-related atrial fibrillation. J. Am. Coll. Cardiol. 2009;54:2075–2083. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Monahan K., Hodges E., Agrawal A., Upender R., Abraham R.L. Signal-averaged P wave area increases during respiratory events in patients with paroxysmal atrial fibrillation and obstructive sleep apnea. Sleep Breath. 2019 doi: 10.1007/s11325-019-01823-5. [DOI] [PubMed] [Google Scholar]
- 37.Mehra R., Stone K.L., Varosy P.D., Hoffman A.R., Marcus G.M., Blackwell T., Ibrahim O.A., Salem R., Redline S. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monahan K., Storfer-Isser A., Mehra R., Shahar E., Mittleman M., Rottman J., Punjabi N., Sanders M., Quan S.F., Resnick H., Redline S. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J. Am. Coll. Cardiol. 2009;54:1797–1804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehra R., Benjamin E.J., Shahar E., Gottlieb D.J., Nawabit R., Kirchner H.L., Sahadevan J., Redline S. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linz D., Brooks A.G., Elliott A.D., Kalman J.M., McEvoy R.D., Lau D.H., Sanders P. Nightly variation in sleep apnea severity as atrial fibrillation risk. J. Am. Coll. Cardiol. 2018;72:2406–2407. doi: 10.1016/j.jacc.2018.08.2159. [DOI] [PubMed] [Google Scholar]
- 41.Linz D., Brooks A.G., Elliott A.D., Nalliah C.J., Hendriks J.M.L., Middeldorp M.E., Gallagher C., Mahajan R., Kalman J.M., McEvoy R.D., Lau D.H., Sanders P. Variability of sleep apnea severity and risk of atrial fibrillation. The VARIOSA-AF study. J. Am. Coll. Cardiol.: Clin. Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Defaye P., Pepin J.L., Poezevara Y., Mabo P., Murgatroyd F., Levy P., Garrigue S. Automatic recognition of abnormal respiratory events during sleep by a pacemaker transthoracic impedance sensor. J. Cardiovasc. Electrophysiol. 2004;15:1034–1040. doi: 10.1046/j.1540-8167.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 43.Defaye P., de la Cruz I., Martin Almor J., Villuendas R., Bru P., Senechal J., Tamisier R., Pepin J.L. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: the DREAM European study. Heart Rhythm. 2014;11:842–848. doi: 10.1016/j.hrthm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Moubarak G., Bouzeman A., de Geyer d'Orth T., Bouleti C., Beuzelin C., Cazeau S. Variability in obstructive sleep apnea: analysis of pacemaker-detected respiratory disturbances. Heart Rhythm. 2017;14:359–364. doi: 10.1016/j.hrthm.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Mazza A., Bendini M.G., De Cristofaro R., Lovecchio M., Valsecchi S., Boriani G. Pacemaker-detected severe sleep apnea predicts new-onset atrial fibrillation. Europace. 2017;19:1937–1943. doi: 10.1093/europace/euw371. [DOI] [PubMed] [Google Scholar]
- 46.O'Hare E., Flanagan D., Penzel T., Garcia C., Frohberg D., Heneghan C. A comparison of radio-frequency biomotion sensors and actigraphy versus polysomnography for the assessment of sleep in normal subjects. Sleep Breath. 2015;19:91–98. doi: 10.1007/s11325-014-0967-z. [DOI] [PubMed] [Google Scholar]
- 47.McDonald K., O'Hanlon R., Savage H.O., Khushaba R.N., Colefax M., Farrugia S., Javed F., Schindhelm K., Wilcox I., Cowie M.R. Sleep-disordered breathing in chronic heart failure is highly variable when measured remotely using a novel non-contact biomotion sensor. Eur. J. Heart Fail. 2017;19:688–690. doi: 10.1002/ejhf.810. [DOI] [PubMed] [Google Scholar]
- 48.Savage H.O., Khushaba R.N., Zaffaroni A., Colefax M., Farrugia S., Schindhelm K., Teschler H., Weinreich G., Grueger H., Neddermann M., Heneghan C., Simonds A., Cowie M.R. Development and validation of a novel non-contact monitor of nocturnal respiration for identifying sleep-disordered breathing in patients with heart failure. ESC Heart Fail. 2016;3:212–219. doi: 10.1002/ehf2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linz D., Kadhim K., Brooks A.G., Elliott A.D., Hendriks J.M.L., Lau D.H., Mahajan R., Gupta A.K., Middeldorp M.E., Hohl M., Nalliah C.J., Kalman J.M., McEvoy R.D., Baumert M., Sanders P. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int. J. Cardiol. 2018;272:155–161. doi: 10.1016/j.ijcard.2018.07.124. [DOI] [PubMed] [Google Scholar]
- 50.Baumert M., Immanuel S.A., Stone K.L., Litwack Harrison S., Redline S., Mariani S., Sanders P., McEvoy R.D., Linz D. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fino E., Mazzetti M. Monitoring healthy and disturbed sleep through smartphone applications: a review of experimental evidence. Sleep Breath. 2019;23:13–24. doi: 10.1007/s11325-018-1661-3. [DOI] [PubMed] [Google Scholar]
- 52.Yoon H., Hwang S.H., Choi S.H., Choi J.W., Lee Y.J., Jeong D.U., Park K.S. Wakefulness evaluation during sleep for healthy subjects and OSA patients using a patch-type device. Comput. Methods Programs Biomed. 2018;155:127–138. doi: 10.1016/j.cmpb.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 53.T. Penzel, C. Schöbel, I. Fietze, New technology to assess sleep apnea: wearables, smartphones, and accessories. F1000Res. 7 (2018) 413. [DOI] [PMC free article] [PubMed]
- 54.Desteghe L., Hendriks J.M.L., McEvoy R.D., Chai-Coetzer C.L., Dendale P., Sanders P., Heidbuchel H., Linz D. The why, when and how to test for obstructive sleep apnea in patients with atrial fibrillation. Clin. Res. Cardiol. 2018;107:617–631. doi: 10.1007/s00392-018-1248-9. [DOI] [PubMed] [Google Scholar]
- 55.Joosten S.A., Hamilton G.S., Naughton M.T. Impact of weight loss management in OSA. Chest. 2017;152:194–203. doi: 10.1016/j.chest.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 56.Middeldorp M.E., Pathak R.K., Meredith M., Mehta A.B., Elliott A.D., Mahajan R., Twomey D., Gallagher C., Hendriks J.M.L., Linz D., McEvoy R.D., Abhayaratna W.P., Kalman J.M., Lau D.H., Sanders P. PREVEntion and regReSsive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 57.Mendelson M., Lyons O.D., Yadollahi A., Inami T., Oh P., Bradley T.D. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur. Respir. J. 2016;48:142–150. doi: 10.1183/13993003.01897-2015. [DOI] [PubMed] [Google Scholar]
- 58.Woehrle H., Ficker J.H., Graml A., Fietze I., Young P., Teschler H., Arzt M. Telemedicine-based proactive patient management during positive airway pressure therapy: impact on therapy termination rate. Somnologie (Berl) 2017;21:121–127. doi: 10.1007/s11818-016-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pepin J.L., Tamisier R., Hwang D., Mereddy S., Parthasarathy S. Does remote monitoring change OSA management and CPAP adherence? Respirology. 2017;22:1508–1517. doi: 10.1111/resp.13183. [DOI] [PubMed] [Google Scholar]