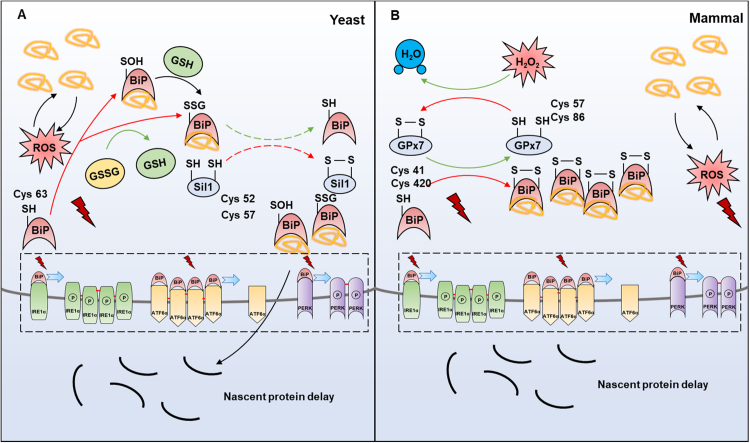
Fig. 6.
Redox regulation of BiP to control UPR. (A) BiP is an ER chaperone that interacts with downstream transducers in the ER. This interaction can be disrupted by the accumulation of misfolded proteins. In yeast, the Cys63 of BiP can be sulfenylated by H2O2, or glutathionylated by GSSG, which enhances its ability to bind to misfolded proteins. Meanwhile, its ATPase activity is downregulated, resulting in the blockage of nascent proteins’ transportation. Sil1 and BiP exchange disulfide, thereby allowing BiP to restore its ATPase activity. (B) In mammals, the redox modification of BiP is mediated by disulfide-bonded GPx7, which oxidizes BiP to form a disulfide bond between its Cys41 and Cys420 residues.