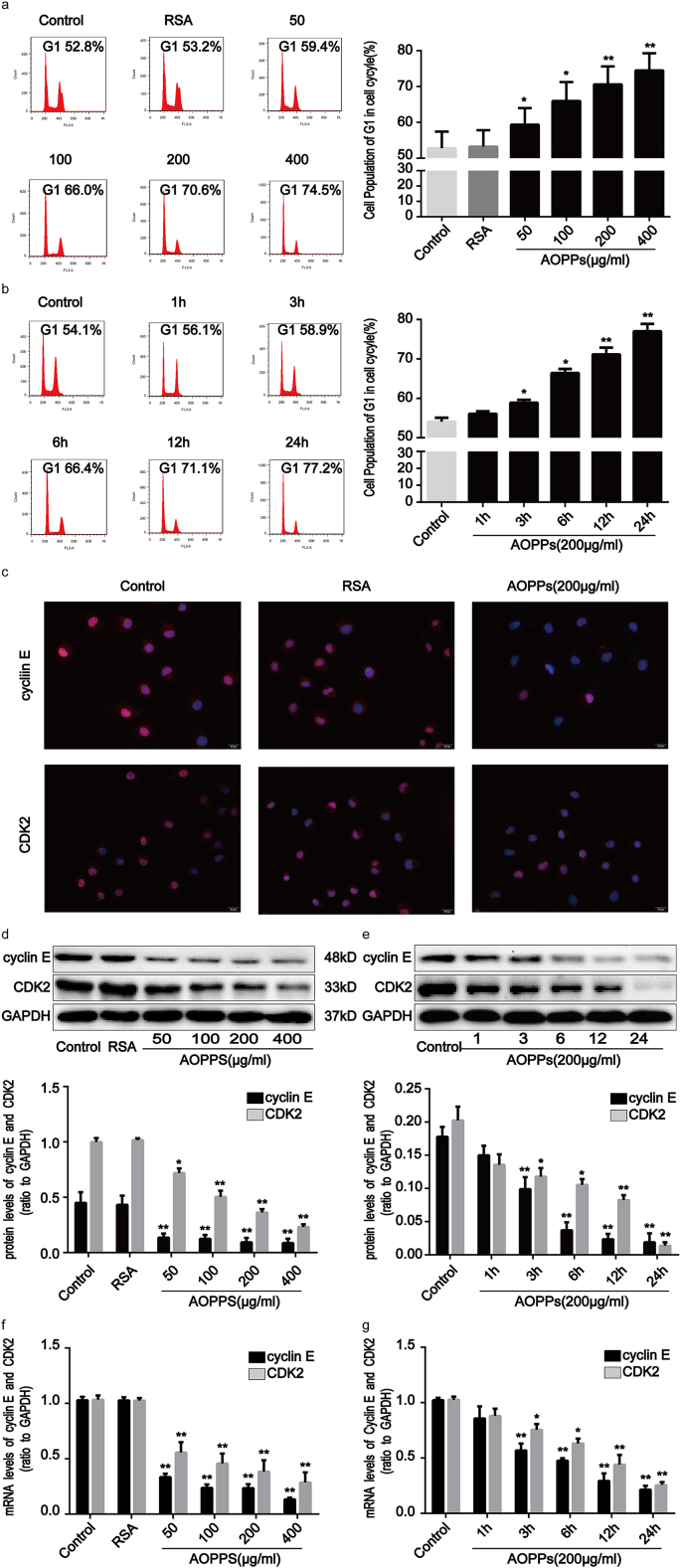
Fig. 2.
AOPPs treatment induced G1 phase arrest in IEC-6 cells in a concentration- and time-dependent manner. IEC-6 cells were treated with control medium, RSA (50 μg/ml), or the indicated concentration of AOPPs for 24 h (a, d, f) or 200 μg/ml of AOPPs at the indicated times (b, e, g), or 200 μg/ml of AOPPs for 24 h (c). Cell cycle distributions were determined by quantitative fluorescence-activated cell sorting (FACS) (a, b). Flow cytometry revealed that a challenge with AOPPs increased the percentage of cells arrested in G1 in a dose- and time-dependent manner. Quantitative analyses of FACS data represent the percentage of G1 phase cells. (c) Immunofluorescence staining for cyclin E and CDK2 revealed that stimulation with AOPPs resulted in a significant reduction of cyclin E and CDK2 in IEC-6 cells. Scale bar, 20 μm. (d, e) Representative Western blotting and bar graphs depicting densitometry quantification showing a reduction of cyclin E and CDK2 protein expression in IEC-6 cells after AOPPs treatment. (f, g) mRNA expression of cyclin E and CDK2 was determined by qPCR. Bar graphs showing qPCR results revealed that AOPPs treatment reduced cyclin E and CDK2 gene expression. Relative protein and mRNA levels of cyclin E and CDK2 were normalized with GAPDH, respectively. Data are presented as mean ± SD from three independent experiments; error bars indicate SD. *P < 0.05 and **P < 0.01 vs control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; qPCR, quantitative real-time polymerase chain reaction.