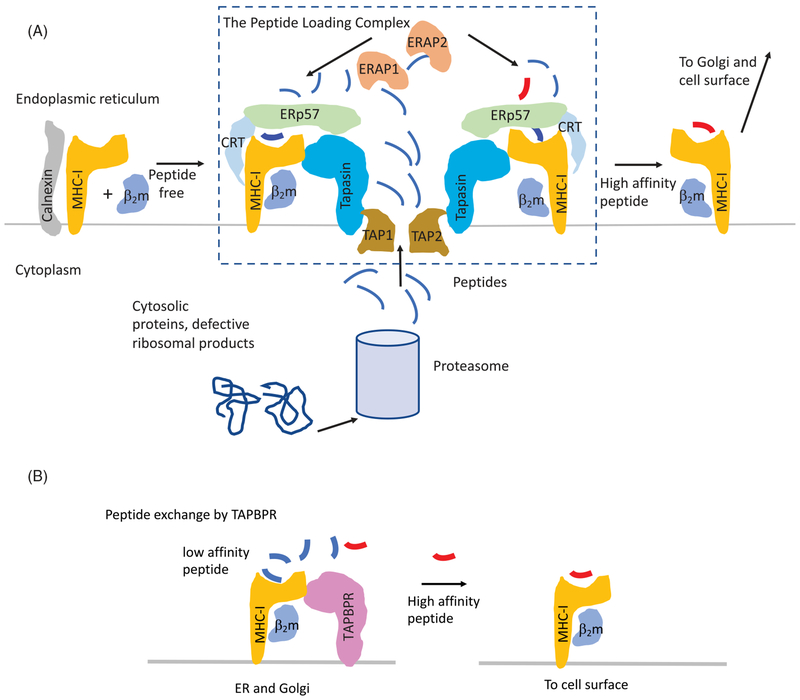
Figure 1.
Schematic representation of peptide-loading steps in the classical (PLC-dependent) and PLC-independent pathways. (A) PLC-dependent pathway. MHC-I heavy chains, folding cotranslationally in the ER, are chaperoned first by calnexin, as the light chain, β2m, coassembles. The MHC-I/β2m heterodimer then joins the peptide-loading complex (PLC) where calnexin is replaced by calreticulin (CRT) that bridges the MHC-I heavy chain to ERp57. CRT additionally interacts with the chaperone tapasin, which is also known as TAP-binding protein (TAPBP) due to its physical association with the TAP transporter. Peptides, generated in the cytoplasm by proteasomal degradation of defective translation, are actively transported via the TAP1/2 heterodimer where they assemble with the MHC-I heavy chain, are trimmed by ERAP1 or ERAP2. High affinity peptides lead to dissociation of the pMHC–I/β2m complex from the rest of the PLC. This complex then proceeds through the Golgi to the cell surface. Steps in glycosylation are not shown. (B) PLC-independent pathway. Low affinity pMHC–I/β2m complexes are bound by TAPBPR, either in the ER or cis-Golgi. Exposure to high affinity peptide leads to dissociation of TAPBPR from the pMHC–I/β2m complex (see colour version of this figure at www.tandfonline.com/ibmg).