Figure 3.
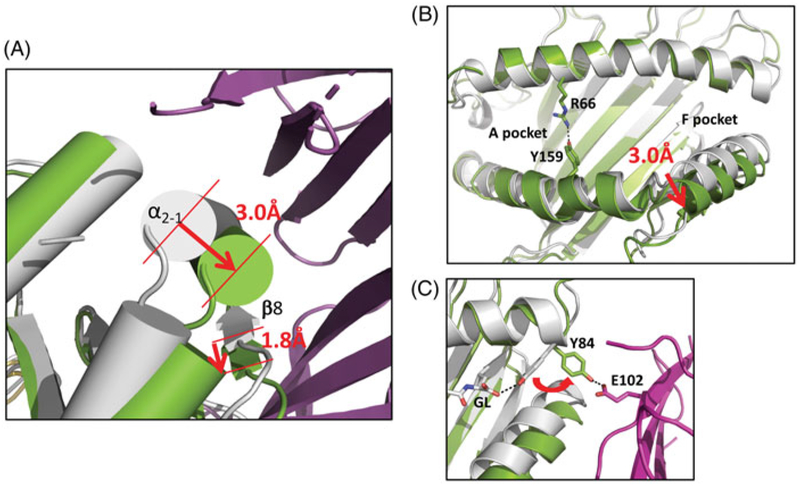
Conformational changes of MHC-I accompany interaction with TAPBPR. (A) α2–1 helix of H2-Dd/5-mer (gray) moves 3.0 Å on interaction with TAPBPR, and platform strand, β8, is displaced by 1.8 Å. (Green indicates H2-Dd in complex with TAPBPR (purple) (B) TAPBPR complexed H2-Dd shows rearrangement of binding groove with closing of A and B pockets and opening of F pocket. (C) TAPBPR interaction rearranges orientation of side chain of Y84 of H2-Dd, a conserved residue that normally coordinates with carboxyl terminus of peptide, forms hydrogen bond to E102 of TAPBPR (see colour version of this figure at www.tandfonline.com/ibmg).