Abstract
Objective
To study the effect of botulinum neurotoxin (BoNT) treatment in jerky and tremulous functional movement disorders (FMD).
Methods
Patients with invalidating, chronic (>1 year) symptoms were randomly assigned to two subsequent treatments with BoNT or placebo every 3 months with stratification according to symptom localisation. Improvement on the dichotomised Clinical Global Impression-Improvement scale (CGI-I) (improvement vs no change or worsening) at 4 months, assessed by investigators blinded to the allocated treatment was the primary outcome. Subsequently all patients were treated with BoNT in a ten month open-label phase.
Results
Between January 2011 and February 2015 a total of 239 patients were screened for eligibility of whom 48 patients were included. No difference was found on the primary outcome (BoNT 16 of 25 (64.0%) vs Placebo 13 of 23 patients (56.5%); proportional difference 0.075 (95% CI −0.189 to 0.327; p=0.77). Secondary outcomes (symptom severity, disease burden, disability, quality of life and psychiatric symptoms) showed no between-group differences. The open-label phase showed improvement on the CGI-I in 19/43 (44.2%) of remaining patients, with a total of 35/43 (81.4%) improvement compared with baseline.
Conclusions
In this double-blind randomised controlled trial of BoNT for chronic jerky and tremulous FMD, we found no evidence of improved outcomes compared with placebo. Motor symptoms improved in a large proportion in both groups which was sustained in the open-label phase. This study underlines the substantial potential of chronic jerky and tremulous FMD patients to recover and may stimulate further exploration of placebo-therapies in these patients.
Trial registration number
NTR2478
Introduction
Despite the fact that functional neurological symptoms (FNS) comprise a third of the patient population of a neurologist, and disease and financial burden on the healthcare system is large, research on optimal treatment in this field has been very limited. In movement disorder clinics functional movement disorders (FMD) account for up to 25% of patients seen.1 2 The diagnosis is based on positive clinical symptoms, supported by neurophysiological tests.2–4 Among FMD, jerks and tremor are frequently seen and have a relatively poor outcome at long-term follow-up (3–7 years).5 6 Botulinum neurotoxin (BoNT) has emerged as a useful therapy for several movement disorders associated with muscle overactivity, including dystonia, and tics.7 8 The mechanism of action of BoNT is more extensive than blocking muscle activity alone; in dystonia for instance there is supporting evidence that BoNT induces plastic changes in the brain.9 Case reports in FMD have reported promising effects of BoNT treatment,6 10 especially in functional dystonia in which large placebo-effects have also been described.11 This has stressed the importance of conducting a controlled clinical trial to disentangle response to an active agent vs placebo. Based on the literature, and our own experience in patients with jerky and tremulous FMD, we hypothesised that BoNT treatment enables restoring the abnormal movement pattern and therefore will be more effective than placebo. To study the long-term effects of BoNT, a subsequent open-label extension study was conducted following the randomised trial phase.
Methods
Study design
This study was designed as a 4-month single-centre, randomised, double-blind, placebo-controlled trial (RCT), followed by an open-label extension phase consisting of up to four treatment sessions for an additional period up to 10 months. BoNT injections were compared with injections with placebo in patients with jerky and tremulous FMD. In the follow-up period, the long-term effects of BoNT injections were assessed.
This study was performed at a tertiary referral centre for movement disorders in the Amsterdam UMC, the Netherlands.
Patients
All eligible patients with jerky and tremulous FMD were consecutively seen at our outpatient clinic from 2000 or specifically referred to us for this study. Included patients had incapacitating functional jerks for at least 1 year, were aged between 18 and 80 years. To improve inclusion, during the study an amendment of the protocol was made adding patients with functional tremor. The diagnosis was made by two experienced movement disorder specialists (JHTMK, MAJT) and symptoms had to fulfil the criteria for ‘definite’ or ‘probable’ FMD.2 No change in medication was allowed in the month prior to participation. Exclusion criteria were pregnancy, coagulation disorders and insufficient knowledge of the Dutch language.
Randomisation and masking
An independent trial nurse, not involved in the treatment or assessment of the outcome measures, carried out a web-based randomisation procedure (ALEA; www.aleaclinical.eu) and prepared the study medication. BoNT is available as powder for injection; after dissolving it is a colourless fluid indistinguishable from sterile saline which was used as placebo. Both BoNT and placebo were prepared in identical syringes. Patients were randomised with a ratio of 1:1 to BoNT or placebo treatment. Randomisation was stratified by localisation of symptoms (extremity vs axial), using permuted blocks with varying block sizes (2 and 4). All patients were treated by the same experienced neurophysiologist (JHTMK). The patients, treating physicians and research group were uninformed about the allocated treatment.
Procedures
Treatment consisted of either intramuscular injections with BoNT type A (BoNT-A) or placebo. Freeze-dried BoNT-A (Dysport, Ipsen BV, Hoofddorp, the Netherlands) was diluted to 20 units (IU) per 0.1 mL of 0.9% sterile saline and aspirated in 1 mL syringes. Placebo consisted of an equivalent volume of 0.9% sterile saline. Injections were given under simultaneous electromyogram recordings into selected muscles using a hollow, Teflon-coated, 27-gauge needle.
During the RCT, all patients were treated twice with either BoNT or placebo; at baseline and 3 months thereafter. The dosages of BoNT were based on the volume of the muscle(s) injected.12 Similar to an RCT in writer’s cramp, the dosage was doubled at the second treatment according to the degree of response.13 After 3 months, all patients subsequently received treatment with BoNT in an open-label extension phase, resulting in a maximum of 4 open-label injections.
At baseline, patients underwent a standardised neurological examination, video recording with a standardised protocol (online appendix 4), and demographic characteristics were gathered. Explanation of the study including efficacy and the most common adverse events of BoNT were given (online appendix 2). All patients underwent electrophysiological examination (polymyography) to support FMD and to select muscles for BoNT treatment.3 In suitable patients, an electroencephalography was added to support FMD (eg, bereitschaftspotential).14
jnnp-2018-320071supp002.pdf (185.4KB, pdf)
Outcomes
The outcome measures were assessed at baseline, at 4 months (primary endpoint) and 4 weeks after the last treatment (end of study). Outcome indicators were: motor symptoms, motor severity, disease burden, muscle weakness, disability, quality of life and quantitative psychiatric assessment. The primary endpoint was improvement of motor symptoms based on the video recordings rated by investigators using the Clinical Global Impression–Improvement (CGI-I) scale (a 7-point scale, ranging from 1=very much improved to 7=very much worse).15 Improvement was defined as a CGI-I score 1, 2 or 3.
The severity of motor symptoms was determined using the Clinical Global Impression–Severity (CGI-S) Scale (a 7-point scale, 1=no symptoms to 7=very severe symptoms) and the Psychogenic Movement Disorder Rating Scale (PMDRS).16 Two investigators (out of nine assessors: JD, JG, EZ, DP, MFC, RZ, BP, AM, JDS) per subset of patients who were blinded to the allocated treatment independently assessed symptom improvement (CGI-I) and severity (CGI-S, PMDRS) based on the video recordings. Ratings of the most experienced rater were used in the final analysis. The second rater served in an inter-observer analysis.
Patients rated their perceived symptom improvement and severity using the CGI-I and CGI-S as well, combined with a 100 mm horizontal Visual Analogue Scale measuring disease burden (0 indicating no disease burden and 100 indicating the worst possible disease burden). Muscle weakness and atrophy were examined objectively by a blinded physician (JHTMK) using the Medical Research Council (MRC) scale. The Academic Medical Center (AMC) Linear Disability Score was used to measure activities of daily life. Quality of life was assessed using the 36-item Short Form Health Survey (SF-36). Quantitative questionnaires concerning psychiatric symptoms were assessed, including the Beck Depression Inventory, Montgomery Asberg Depression Rating Scale, Beck Anxiety Inventory, Liebowitz Social Anxiety Scale, Obsessive Compulsive Inventory (online appendix 1).
The most common adverse events of BoNT (pain, weakness, influenza-like symptoms and any other negative effects) were actively asked for. During the trial phase, patients were asked whether they thought they received BoNT or placebo, how much confidence they had in the treatment beforehand and whether they wanted to continue treatment.
Statistical analysis
We assumed that 30% of patients in the placebo group and 70% of the patients of the BoNT group would reach the primary endpoint. The placebo effect was hypothesised to be larger than the effect size found in a similar BoNT trial in focal dystonia (writer’s cramp).13 A two-group Χ2 test with a 0.05 two-sided significance level generated a sample size of 24 patients per arm with a power of 80%.
All analyses were done according to the intention-to-treat principle. The baseline characteristics, outcome parameters and (serious) adverse events were summarised using descriptive statistics. The CGI-I was dichotomised to improvement (score 1,2 or 3) vs no change or worsening (score 4,5,6,7). Between-group differences in proportions (trial phase) were assessed using the χ2 test or Fisher’s exact test, when appropriate. Statistical uncertainty was expressed in a two-sided 95% CI. With regard to the primary outcome indicator, we additionally performed multivariable logistic regression with treatment groups and the stratification factor (axial vs extremity) as independent variables. The effect size was expressed in an adjusted OR.
As most continuous secondary outcome measures were non-normally distributed, all outcome measures were described in median scores, with their IQR).
The within-group median change scores (from baseline to outcome assessment) in the trial phase were calculated as the 50th percentile of all individual differences. Point estimate and 95% CI of the median differences in change scores between the treatment groups was analysed using the Hodges-Lehmann approach.17 Between-group difference in change scores was also analysed using the Mann-Whitney U test.
The difference in within-group median scores in the open-label extension phase was analysed with the Wilcoxon signed-rank test for paired data.
Interobserver agreements of the ordinal ratings on the CGI-I and CGI-S were analysed using the average weighted Kappa (K) statistic. With regard to the interobserver agreements of the continuous PMDRS scores, we used the average intraclass correlation coefficient (ICC) (online appendix 2).
A two-sided p value <0.05 was considered statistically significant. Missing data were not imputed and no interim analysis was performed. All analyses were performed in IBM SPSS Statistics V.24 and STATA V.15. The pre-defined statistical analysis plan is available in online appendix 3.
Post-hoc analyses
The primary outcome measure was analysed setting a higher bar of improvement (CGI-I score 1–2 vs 3–7). Possible predictors of treatment outcome on the CGI-I were analysed using a logistic regression including: symptom duration, psychiatric co-morbidity (anxiety/depressive disorder), quantitative pain measures (subscale SF-36), pain disorder, confidence in treatment beforehand and which treatment arm patients thought they were in.
Classification of evidence
The aim of this was to provide class I evidence according to the classification of evidence from the American Academy of Neurology18 19 on the effect of BoNT treatment in patients with jerk-like FMD.
Standard protocol approvals, registrations and patient consents
The trial is registered in the Dutch Trial Register (NTR 2478) (http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2478) and was monitored by an independent monitor of the Amsterdam University Medical Center (UMC), according to Good Clinical Practice (GCP) guidelines. The study protocol can be found online (https://www.amc.nl/web/specialismen/neurologie-1/botulinum-neurotoxin-bont-trial.htm).
Results
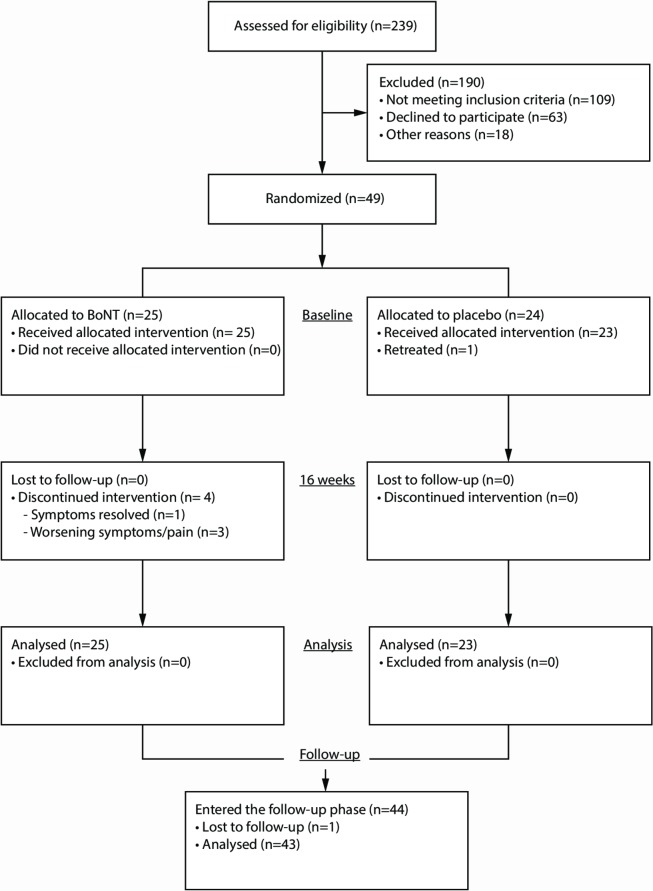
Between 27 January 2011 and 18 February 2015 a total of 239 patients were screened for eligibility of whom 49 patients were randomised and 48 actually received treatment (figure 1). For details on reasons of exclusion see online supplementary appendix 2. Twenty-five patients were treated with BoNT and 23 patients with placebo. All patients were BoNT-naïve except for one patient who was treated ineffectively once before. He was considered to possibly benefit from treatment with better muscle selection using polymyography. All patients completed the trial phase of the study. The baseline characteristics are summarised in table 1. The majority of patients in both treatment arms underwent previous other sorts of treatments. Examples of included patients reflecting the clinical spectrum are shown in online videos 1-3.
Figure 1.
Study flow chart: enrolment, randomisation and follow-up of patients. For details on reasons why patients did not fulfil the inclusion criteria, see online appendix 2. BoNT, botulinum neurotoxin.
Table 1.
Baseline characteristics of study population.
| BoNT n=25 | Placebo n=23 | |
| Age, year (median; IQR) | 50.0 (40.0; 61.5) | 54.0 (37.0; 57.0) |
| Gender, n (%) | ||
| Male | 14 (56) | 14 (61) |
| Duration of disease, year (median; IQR) | 5.0 (2.1; 13.4) | 5.3 (2.2; 9.0) |
| Fahn and Williams diagnostic criteria, n (%) | ||
| Clinically definite | 18 (72.0) | 16 (69.6) |
| Clinically probable | 7 (28.0) | 7 (30.4) |
| Additional phenomenology based on PMDRS, n (%) | ||
| Dystonia | 12 (48.0) | 10 (43.5) |
| Tic | 3 (12.0) | 2 (8.7) |
| Distribution of jerks/tremor, n (%) | ||
| Abdomen | 12 (48.0) | 11 (47.8) |
| Extremity | 13 (52.0) | 12 (52.2) |
| Education level, n (%) | ||
| Primary school | 1 (4.0) | 2 (8.7) |
| Lower education | 4 (16.0) | 3 (13.0) |
| Medium education/higher school | 11 (44.0) | 12 (52.2) |
| Higher education/university | 9 (36.0) | 6 (26.1) |
| Unemployed | 17 (68.0) | 13 (56.5) |
| Disease-related* | 13 (52.0) | 11 (47.8) |
| Clinical neurophysiology, n (%)† | 13 (52.0) | 14 (60.9) |
| Pre-movement potential | 11 (61.5) | 11 (57.1) |
| Previous treatment, n (%)‡ | 21 (84.0) | 22 (95.7) |
| Rehabilitation | 4 (16.0) | 5 (21.7) |
| Physiotherapy | 8 (34.8) | 9 (36.0) |
| Psychotherapy | 5 (20.0) | 7 (30.4) |
| Other§ | 17 (68.0) | 17 (73.9) |
*Other forms of financial income included retirement (n=4 BoNT vs n=1 placebo), study (n=1 placebo).
†EEG-EMG with backaveraging could not be performed in the whole population.
‡More than one category could apply per patient.
§Other treatment includes acupuncture, homeopathic treatment, alternative medicine, hypnosis, benzodiazepines, Selective Serotonin Reuptake Inhibitors (SSRIs), anti-epileptics, dopamine-agonists.
BoNT, botulinum neurotoxin; EEG, electroencephalogram; EMG, electromyogram; PMDRS, Psychogenic Movement Disorder Rating Scale.
Trial phase
Due to pain/worsening symptoms (n=2) and complete resolution of symptoms (n=1), 3 patients discontinued BoNT treatment after one injection. One patient discontinued the study after two BoNT injections because of pain (figure 1). In one patient, assessment of the primary endpoint was delayed to 1 year instead of 4 months due to personal circumstances.
In the BoNT arm the median initial dose was 240 IU (IQR 140–400) followed by 440 IU (IQR 240–705) at the second visit. The iliopsoas (n=8), rectus abdominis (n=7) and quadriceps muscle (n=6) were most frequently injected. In the placebo group patients were treated with a volume of sterile saline equivalent to a median dose of 280 IU (IQR 130–400) at the first visit, and 450 IU (IQR 240–640) at the second visit; the rectus abdominis (n=5) and iliopsoas muscle (n=5) were most frequently injected. Usually two muscles per subject were injected (range 1–6). For an extensive overview of the injected muscles and corresponding doses see online appendix 2.
The interobserver agreement was substantial for the CGI-I and CGI-S (average weighted κ=0.65, SD 0.16), and the PMDRS (average ICC=0.76, SD 0.11). At the end of the trial-period 9 out of 25 (36.0%) patients in the BoNT vs 8 out of 22 (34.8%) patients in the placebo arm thought they received BoNT. Three (12.0%) patients in the BoNT vs 1 (4.3%) in the placebo arm could not answer the question.
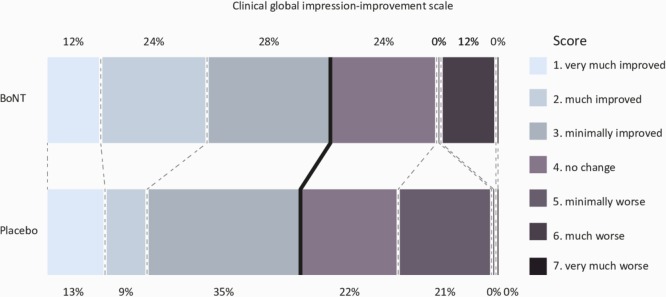
Regarding the primary outcome measure; 16 of 25 (64.0%) patients in the BoNT arm showed improvement of motor symptoms (CGI-I score 1, 2 or 3) compared with 13 of 23 (56.5%) in the placebo arm (figure 2). This resulted in a non-significant proportional difference of 0.075 (95% CI −0.189 to 0.327; p=0.77). Multivariable logistic regression analysis, adjusted for the stratification variable (extremity vs axial), also did not reveal an effect of BoNT (adjusted OR 1.371; 95% CI 0.428 to 4.390; p=0.60).
Figure 2.
Distribution of scores of the primary outcome (Clinical Global Impression-Improvement scale). The thick black line indicates the cut-off point of improvement (score 1, 2 or 3) vs no change or worsening (score 4, 5, 6 and 7). No significant difference was found between the two treatment arms (BoNT vs placebo; proportional difference of 0.075 (95% CI −0.189 to 0.327; p=0.77)). BoNT, botulinum neurotoxin.
The CGI-I rated by patients (secondary endpoint), showed a perceived improvement (score 1, 2 or 3) in 12 out of 25 (48.0%) patients of the BoNT arm compared with 12 out of 23 (52.2%) in the placebo arm resulting in a non-significant proportional difference of −0.042 (95% CI −0.300 to 0.225; p=1.00). The other secondary outcome measures are summarised in table 2. There were marginal and non-significant median differences in change scores between the BoNT and placebo group.
Table 2.
Secondary outcome measures the end of the trial.
| BoNT (n=25) | Placebo (n=23) | Treatment comparison | ||||||
| Median score (IQR) at baseline | Median score (IQR) end of trial | Median score (IQR) change score | Median score (IQR) at baseline | Median score (IQR) end of trial | Median score (IQR) change score | Median difference in change scores (95% CI) | P value* | |
| CGI-Severity investigator | 3.0 (2.0; 5.0) | 3.0 (2.0; 4.0) | - 1.0 (−1.0; 0.5) | 4.0 (3.0; 5.0) | 4.0 (2.0; 5.0) | 0.0 (−1.0; 0.0) | −1.0 (−1.0 to 1.0) | 0.821 |
| CGI-Severity patient | 5.0 (4.0; 6.0) | 4.0 (3.0; 6.0) | 0.0 (−1.5; 1.0) | 5.0 (4.0; 6.0) | 4.0 (4.0; 5.0) | −1.0 (−1.0; 0.0) | 1.0 (−1.0 to 1.0) | 0.799 |
| VAS-disease burden patient | 49.0 (30.5; 71.0) | 34.0 (14.0; 78.5) | −2.0 (−27.5; 20.0) | 62.0 (40.0; 86.0) | 48.0 (29.0; 67.0) | −2.0 (−48.0; 12.0) | 0.0 (−25.0 to 15.0) | 0.613 |
| PMDRS-motor symptoms | 10.0 (5.0; 18.0) | 8.0 (4.5; 15.0) | −3.0 (−6.5; 2.5) | 17.0 (10.0; 21.0) | 16.0 (9.0; 22.0) | 0.0 (−5.0; 2.0) | −3.0 (−2.0 to 4.0) | 0.438 |
| SF-36-Physical component | 37.8 (24.3; 54.4) (n=24) |
36.3 (24.0; 55.1) (n=22) |
−1.2 (−5.9; 4.4) | 33.2 (26.7; 42.1) | 32.6 (27.1; 43.0) | −1.3 (−3.7; 2.2) | 0.1 (−4.2 to 4.2) | 0.964 |
| SF-36-Mental component | 50.8 (41.1; 57.9) (n=24) |
52.3 (41.2; 55.4) (n=22) |
0.2 (−5.6; 3.8) | 52.9 (44.0; 60.3) | 52.5 (40.0; 60.6) | 1.0 (−2.0; 6.4) | −0.8 (−3.6 to 5.4) | 0.768 |
| ALDS-disability | 88.4 (84.2; 89.5) (n=24) |
89.5 (78.8; 89.5) (n=22) |
0.0 (−2.5; 0.1) | 87.4 (79.0; 89.5) | 86.9 (79.3; 89.2) | −0.3 (−2.1; 0.0) | 0.3 (−1.3 to 1.6) | 0.624 |
| BDI-Depressive symptoms | 8.0 (4.5; 14.0) (n=24) |
8.5 (4.5; 14.0) (n=22) |
−1.0 (−4.0; 3.3) | 9.0 (5.0; 13.0) | 10.0 (3.0; 11.0) | 0.0 (−3.0; 1.0) | −1.0 (−3.0 to 3.0) | 0.802 |
| MADRS-Depressive symptoms | 12.0 (5.0; 15.5) | 9.0 (4.0; 16.0) | 0.0 (−3.0; 3.0) | 13.0 (6.0; 18.0) | 13.0 (8.0; 22.0) | 3.00 (−4.0; 7.0) | −3.0 (−1.0 to 6.0) | 0.214 |
| BAI-Anxiety symptoms | 10.0 (6.3; 15.8) (n=24) |
10.0 (2.8; 15.3) (n=24) |
1.0 (−1.5; 3.0) | 13.0 (8.0; 18.0) | 12.0 (5.0; 18.0) | 0.0 (−5.0; 2.0) | 1.0 (−5.0 to 1.0) | 0.213 |
| LSAS-Social anxiety | 10.0 (5.3; 27.0) (n=24) |
10.5 (4.8; 20.8) (n=22) |
−2.5 (−6.3; 5.0) | 10.0 (3.5; 17.5) (n=22) |
9.0 (4.0; 25.0) | 0.0 (−4.0; 8.3) | −2.5 (−4.0 to 10.0) | 0.264 |
| OCI-Obsessive-Compulsive symptoms | 2.0 (0.3; 6.8) (n=24) |
4.0 (1.0; 6.3) (n=24) |
0.0 (−1.0; 3.3) | 2.0 (0.0; 5.0) | 4.0 (1.0; 7.0) | 1.0 (0.0; 3.0) | 1.0 (-1.0 to 2.0) | 0.300 |
The missing data should be noticed.
*Mann-Whitney U test.
ALDS, AMC Linear Disability Score; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CGI, Clinical Global Impression; 95% CI, 95% Confidence Interval (Hodges-Lehmann approach); LSAS, Liebowitz Social Anxiety Scale; MADRS, Montgomery Asberg Depression Rating Scale; OCI, Obsessive Compulsive Inventory; PMDRS, Psychogenic Movement Disorder Rating Scale; SF-36, Short Form 36; VAS, Visual Analogue Scale.
The post-hoc analysis using a higher cut-off point for improvement on the CGI-I (score 1–2 vs 3–7) did not alter our results (p=0.349). Also, no significant predictors for treatment response were found.
Open-label phase
After completing the trial, 44 of 48 patients participated in the open-label phase. Twenty-seven (61.4%) of 44 patients completed four treatment sessions in the open-label study (figure 1).
The median dose administered per visit was 350 IU (IQR 200–480). Usually, two muscles were injected (ranging from 1 to 8). Due to a mistake made by the pharmacy, one patient received placebo instead of BoNT during the first session of the open-label extension study. He was called back and treated again.
Two (4.5 %) patients were treated once, 9 (20.5%) patients twice and 6 (13.6%) patients were treated three times. In the final analysis one patient, who refused to cooperate was lost to follow-up.
Improvement (score 1, 2 or 3) of motor symptoms on the CGI-I assessed by the investigators occurred in 19 of 43 patients (44.2%) compared with the end of the randomised trial (see figure 3), resulting in a total of 35 out of 43 patients (81.4%) showing improvement compared with baseline.
Figure 3.
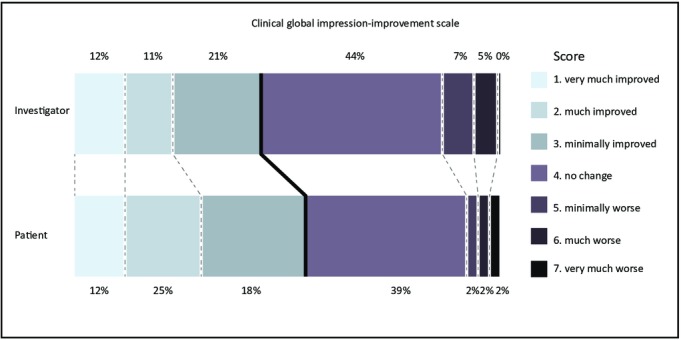
Distribution of the Clinical Global Impression-Improvement Scale scores, assessing improvement of symptoms from the end of trial to the end of the open-label study, scored by the investigator and the patient.
The CGI-I assessed by the patient revealed a perceived motor improvement in 24 of 43 patients (55.8%) compared with the end of the randomised trial (see figure 3), and in 29 of 43 patients (67.4%) compared with baseline (for detailed CGI-I scores see online appendix 2). The other outcome measures are summarised in table 3.
Table 3.
Motor symptoms, disease burden, quality of life, disability and psychiatric outcome at the end of the open-label follow-up
| Median score (IQR) at start open-label phase | Median score (IQR) end of follow-up | P value* | |
| CGI-severity investigator | 3.0 (2.0; 4.0) | 2.0 (1.0; 4.0) | 0.005 |
| CGI-severity patient | 4.0 (4.0; 5.75) | 4.0 (2.0; 5.0) | 0.044 |
| VAS-disease burden patient | 48.0 (20.3; 71.8) | 28.0 (3.0; 62.0) | 0.042 |
| PMDRS-motor symptoms | 10.0 (5.0; 21.0) | 9.0 (3.0; 17.0) | 0.010 |
| SF-36-Physical component† | 34.4 (25.1; 48.3) | 40.2 (27.4; 53.6) | 0.058 |
| SF-36-Mental component† | 52.5 (41.2; 56.9) | 50.0 (37.9; 55.7) | 0.751 |
| ALDS-disability† | 87.8 (79.2; 89.5) | 89.1 (83.5; 89.5) | 0.790 |
| BDI-Depressive symptoms† | 9.0 (4.0; 12.0) | 6.0 (3.0; 13.5) | 0.370 |
| MADRS-Depressive symptoms | 12.0 (15.0; 18.8) | 10.0 (4.0; 18.0) | 0.205 |
| BAI-Anxiety symptoms† | 11.0 (3.5; 17.0) | 7.0 (4.0; 16.0) | 0.127 |
| LSAS-Social anxiety† | 10.0 (4.0; 21.5) | 10.0 (2.0; 16.5) | 0.446 |
| OCI-Obsessive-Compulsive symptoms† | 4.0 (1.0; 7.0) | 3.0 (1.5; 5.0) | 0.028 |
Bold figures indicate P values< 0.05.
*Wilcoxon signed-rank test for paired data.
†Data of n=41 patients.
ALDS, AMC Linear Disability Score; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CGI, Clinical Global Impression; IQR, Interquartile range; LSAS, Liebowitz Social Anxiety Scale; MADRS, Montgomery Asberg Depression Rating Scale; OCI, Obsessive Compulsive Inventory; PMDRS, Psychogenic Movement Disorder Rating Scale; SF-36, Short Form 36; VAS, Visual Analogue Scale.
Of the 44 patients who received open-label treatment, 17 (38.6%) continued treatment with BoNT after the study had ended. These were all patients with relapse of symptoms at the end of every 3 months. In 6 (13.6%) out of 44 patients symptoms diminished/resolved and no further treatment was needed. Of the remaining patients, 15 (34.1%) did not benefit enough, 4 (9.1%) experienced too much side effects, 1 (2.3%) had worsening of symptoms and 1 (2.3%) could not continue treatment because of financial reasons.
Safety
For an overview of all adverse events during the study see table 4. During the trial phase equal proportions of patients per group had adverse events: BoNT 21/25 (84.0%) vs placebo n=20/23 (87.0%). Serious adverse events occurred in two patients (n=1 BoNT; ketamine infusion for chronic pain vs n=1 placebo; hospital admittance for cardial syncope). During the open-label phase serious adverse events occurred in five patients (lumbar disc herniation surgery (n=1), admittance rehabilitation clinic (n=2), ketamine infusion for chronic pain (n=1), surgery de Quervain’s disease (n=1)). All serious adverse events were deemed not related to the study intervention and reported to the local medical ethics committee.
Table 4.
Number of patients with adverse events; patients could fulfil more than one category
| Trial-phase | Open-label phase | ||
| BoNT n=25 | Placebo n=23 | n=43 | |
| Pain injection site, n (%) | 9 (36.0) | 2 (8.7) | 9 (20.9) |
| Haematoma injection site, n (%) | 2 (8.0) | 2 (8.7) | 2 (4.7) |
| Influenza-like symptoms, n (%) | 2 (8.0) | 4 (17.4) | 4 (9.3) |
| Muscle weakness, n (%) | 6 (24.0) | 4 (17.4) | 12 (27.9) |
| MRC scale, median (IQR) | 5 (5; 5) | 5 (5; 5) | 5 (5; 5) |
| Worsening symptoms, n (%) | 5 (20.0) | 3 (13.0) | 3 (7.0) |
| Other*, n (%) | 9 (36.0) | 10 (43.5) | 19 (44.2) |
*Other adverse events included musculoskeletal pain, planned surgery/medical intervention, muscle cramps, infection/inflammation, nausea, stomach ache, diarrhoea, chest pain, shortness of breath, dizziness, memory problems, transient confusion, globus feeling, skin abnormalities, headache and fatigue.
BoNT, botulinum neurotoxin.
This study provides class I evidence that BoNT treatment in jerky and tremulous FMD is not effective compared with placebo.
Discussion
In this 4-month randomised placebo-controlled double-blinded clinical trial, the effect of BoNT treatment on jerky and tremulous FMD was evaluated. Overall, BoNT was safe and well tolerated. We could not demonstrate benefit of BoNT over placebo injections in terms of symptom improvement and severity, disease burden, quality of life, disability and psychiatric symptoms. At the end of the trial phase, motor symptoms of approximately 60% of patients across both treatment conditions improved; 44% of patients showed additional improvement at the end of the 1-year open-label study compared with the end of the trial. Eventually, 81% of patients improved compared with baseline.
Although we assumed the placebo effect to be larger than in a similar trial in writer’s cramp,13 we didn’t anticipate the placebo effect to be this large (57%) as most patients had long-lasting symptoms and have had several other treatments before. Other randomised treatment studies in FNS are scarce and concern physiotherapy,20 rehabilitation,21 disease education,22 23 psychotherapy24 and transcranial magnetic stimulation (TMS).25 Most are not placebo-controlled and the majority reveals significant and clinically relevant improvement in a large proportion of patients (30%–70%). One study with a placebo-like control condition25 (TMS vs spinal cord stimulation) found large effect sizes (66%) in both the intervention and control arm, which is in line with our findings. This suggests treatment effects in FNS are largely due to placebo-effects and the ‘rituals’ accompanying receiving any treatment.26 27 The more invasive, the more effective a placebo therapy may be.28Although it was not the purview of this study, comparing BoNT and placebo to a less invasive therapy (eg, massage, explanation) could have given more insight in this matter.
The lack of effect of BoNT compared with placebo in jerky and tremulous FMD cannot be generalised to other forms of FMD. Organic dystonia is the movement disorder most commonly treated with BoNT. We chose jerky and tremulous FMD because these are more common than functional dystonia1 2 and the diagnostic process can be aided by neurophysiologic tests.4 Effective treatment of functional (fixed) dystonia in a small group with subclinical amounts of BoNT has been described.11 The immediate improvement after injections in this study suggests a placebo-effect. Further, a recent small trial (n=10) in functional dystonia showed no benefit of BoNT over placebo prior to cognitive behavioural therapy.29 In general, it is still debated whether the treatment of FNS should be equalised or specialised to specific motor phenotypes.30 Based on our study and the literature definite conclusions cannot be drawn but BoNT and placebo appears to be as effective in both jerky/tremulous as dystonic FMD.
Apart from the large placebo response, an additional effect of BoNT cannot be excluded. In the open-label extension phase a large proportion of patients still improved compared with the blinded phase. Future dose and treatment duration-finding studies using larger study groups may help to pick up smaller effects. However, given the results of our study it seems more relevant to focus on placebo-like therapies.
Our study population included chronically ill patients (median symptom duration of 5 years) in whom, according to the literature, prognosis is often poor (on average 39% of patients display persistent or worsening symptoms).5 The proportion of patients which improved is therefore a remarkable finding. Notably, this did not translate into amelioration of disability, quality of life and psychiatric symptoms. Unfortunately we could not identify any traits (eg, pain or fatigue) of treatment-responders or non-responders in our post-hoc analyses, which should be interpreted with caution given the small study population. Ultimately, a substantial part of patients (n=17) chose to continue BoNT treatment after the study, suggesting that patients did perceive benefit of the treatment.
The amount of patients included was the result screening of a large number of eligible patients (48 (20%) of 239 patients screened). This is in line with other studies of FNS,20 22 23 but questions the generalisability of the results to all jerky and tremulous FMD patients. However, of the 109 excluded patients the reason was clinically relevant as in almost half of them symptoms diminished severely or resolved, in about a quarter the symptoms were not amendable for injections, and of the remaining quarter there were other reasons including a non-functional disorder. Another interesting observation in our population was the equal number of men and women, not only in the study population, but also in the large screening pool. Axial jerks do tend to occur more often in men,6 so this might be an explanation of our population not reflecting the usual demographics of FMD with a female predominance.
Limitations
Limitations of this study include the small study population and the large number of eligible patients which had to be screened in order to reach the required amount of patients. Also, the patient who was analysed after 1 year instead of 4 months was a major protocol violation.
Conclusions
In this RCT of BoNT for jerky and tremulous FMD, we found no evidence of improved outcomes in patients treated with BoNT compared with placebo. The response to placebo, however, was very large. Our study underlines the potential of patients with chronic FMD to improve. Despite the possible ethical issues, we advocate further exploration of placebo-like therapies in FMD.
jnnp-2018-320071supp001.pdf (41KB, pdf)
jnnp-2018-320071supp003.wmv (25.5MB, wmv)
jnnp-2018-320071supp004.wmv (38.7MB, wmv)
jnnp-2018-320071supp005.wmv (43.9MB, wmv)
Acknowledgments
We would like to thank all the staff members of the department of clinical neurophysiology for their support with carrying out this study. Also we would like to thank dr. Farrah Mateen for her help in improving and editing the manuscript.
Footnotes
Contributors: MAJT, JMD and JHTM designed the study. YEMD and JHTM collected data. YEMD, MAJT, JMD, JHTM and RJdH analysed and interpreted data. YEMD wrote the paper and designed the figures and tables. JMG, EZ, DvP, MFC, RZ, BP, AGM and JDS assessed the primary outcome based on video-tapes of the patients. All other authors drafted and critically revised the paper.
Funding: This study was funded by Prinses Beatrix fund and Ipsen.
Disclaimer: The funders (Prinses Beatrix Fund, Ipsen) were not involved in the study design, data collection, data analysis, data interpretation or writing of the manuscript.
Competing interests: YEMD, JHTMK and MAJT report grants from Prinses Beatrix Fund, non-financial support from Ipsen, during the conduct of the study. JHTMK reports grants from Ipsen, grants from Allergan, grants from Merz, outside the submitted work. MAJT reports grants from Gossweiler Foundation, grants from Phelps Stichting, grants from Stichting wetenschapsfonds dystonie vereniging, grants from Fonds NutsOhra, grants from Ipsen, Allergan, Merz, Actelion, grants from SNN Kennisontwikkeling, European funding, grants from Fonds psychische Gezondheid, outside the submitted work. All other authors have nothing to disclose. The authors vouch for the accuracy and completeness of the data and for the fidelity of this report to the study protocol.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the local medical ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Factor SA, Podskalny GD, Molho ES. Psychogenic movement disorders: frequency, clinical profile, and characteristics. Journal of Neurology, Neurosurgery & Psychiatry 1995;59:406–12. 10.1136/jnnp.59.4.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shill H, Gerber P. Evaluation of clinical diagnostic criteria for psychogenic movement disorders. Movement Disorders 2006;21:1163–8. 10.1002/mds.20921 [DOI] [PubMed] [Google Scholar]
- 3. Schwingenschuh P, Saifee TA, Katschnig-Winter P, et al. . Validation of “laboratory-supported” criteria for functional (psychogenic) tremor. Mov Disord. 2016;31:555–62. 10.1002/mds.26525 [DOI] [PubMed] [Google Scholar]
- 4. Beudel M, Zutt R, Meppelink AM, et al. . Improving neurophysiological biomarkers for functional myoclonic movements. Parkinsonism Relat Disord 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gelauff J, Stone J, Edwards M, et al. . The prognosis of functional (psychogenic) motor symptoms: a systematic review. Journal of Neurology, Neurosurgery & Psychiatry 2014;85:220–6. 10.1136/jnnp-2013-305321 [DOI] [PubMed] [Google Scholar]
- 6. van der Salm SMA, Erro R, Cordivari C, et al. . Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology 2014;83:1862–70. 10.1212/WNL.0000000000000982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cocco A, Albanese A. Recent developments in clinical trials of botulinum neurotoxins. Toxicon 2017. [DOI] [PubMed] [Google Scholar]
- 8. Marras C, Andrews D, Sime E, et al. . Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology 2001;56:605–10. 10.1212/WNL.56.5.605 [DOI] [PubMed] [Google Scholar]
- 9. Hallett M. Mechanism of action of botulinum neurotoxin: unexpected consequences. Toxicon 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erro R, Edwards MJ, Bhatia KP, et al. . Psychogenic axial myoclonus: clinical features and long-term outcome. Parkinsonism Relat Disord 2014;20:596–9. 10.1016/j.parkreldis.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 11. Edwards MJ, Bhatia KP, Cordivari C. Immediate response to botulinum toxin injections in patients with fixed dystonia. Mov. Disord. 2011;26:917–8. 10.1002/mds.23562 [DOI] [PubMed] [Google Scholar]
- 12. Moore AP, Naumann M. Handbook of botulinum toxin treatment. 2nd edn Oxford: Blackwell Science Ltd, 2003. [Google Scholar]
- 13. Kruisdijk JJM, Koelman JHTM, Ongerboer de Visser BW, et al. . Botulinum toxin for writer's cramp: a randomised, placebo-controlled trial and 1-year follow-up. Journal of Neurology, Neurosurgery & Psychiatry 2007;78:264–70. 10.1136/jnnp.2005.083170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apartis E. Clinical neurophysiology of psychogenic movement disorders: how to diagnose psychogenic tremor and myoclonus. Neurophysiologie Clinique/Clinical Neurophysiology 2014;44:417–24. 10.1016/j.neucli.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 15. W G. Clinical Global Impression ECDEU assessment manual for psychopharmacology, revised. National Institute of mental health 1976.
- 16. Hinson VK, Cubo E, Comella CL, et al. . Rating scale for psychogenic movement disorders: Scale development and Clinimetric testing. Mov. Disord. 2005;20:1592–7. 10.1002/mds.20650 [DOI] [PubMed] [Google Scholar]
- 17. Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann. Math. Statist. 1963;34:598–611. 10.1214/aoms/1177704172 [DOI] [Google Scholar]
- 18. Ashman EJ, Gronseth GS. Level of evidence reviews: three years of progress. Neurology 2012;79:13–14. 10.1212/WNL.0b013e31825dce83 [DOI] [PubMed] [Google Scholar]
- 19. Neurology AAo Clinical practice guideline process manual. The American Academy of Neurology 2011. [Google Scholar]
- 20. Nielsen G, Buszewicz M, Stevenson F, et al. . Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017;88:484–90. 10.1136/jnnp-2016-314408 [DOI] [PubMed] [Google Scholar]
- 21. Jordbru AA, Smedstad LM, Klungsøyr O, et al. . Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. J Rehabil Med 2014;46:181–7. 10.2340/16501977-1246 [DOI] [PubMed] [Google Scholar]
- 22. Pleizier M, de Haan RJ, Vermeulen M. Management of patients with functional neurological symptoms: a single-centre randomised controlled trial. J Neurol Neurosurg Psychiatry 2017;88:430–6. 10.1136/jnnp-2015-312889 [DOI] [PubMed] [Google Scholar]
- 23. Sharpe M, Walker J, Williams C, et al. . Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77:564–72. 10.1212/WNL.0b013e318228c0c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kompoliti K, Wilson B, Stebbins G, et al. . Immediate vs. delayed treatment of psychogenic movement disorders with short term psychodynamic psychotherapy: randomized clinical trial. Parkinsonism Relat Disord 2014;20:60–3. 10.1016/j.parkreldis.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 25. Garcin B, Mesrati F, Hubsch C, et al. . Impact of transcranial magnetic stimulation on functional movement disorders: cortical modulation or a behavioral effect? Front Neurol 2017;8 10.3389/fneur.2017.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron 2008;59:195–206. 10.1016/j.neuron.2008.06.030 [DOI] [PubMed] [Google Scholar]
- 27. Benedetti F. Placebo-induced improvements: how therapeutic rituals affect the patient's brain. J Acupunct Meridian Stud 2012;5:97–103. 10.1016/j.jams.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 28. Jonas WB, Crawford C, Colloca L, et al. . To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open 2015;5:e009655 10.1136/bmjopen-2015-009655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vizcarra JA, Lopez-Castellanos JR, Dwivedi AK, et al. . OnabotulinumtoxinA and cognitive behavioral therapy in functional dystonia: a pilot randomized clinical trial. Parkinsonism & Related Disorders 2019. (published Online First: 2019/02/20). 10.1016/j.parkreldis.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 30. Espay AJ, Aybek S, Carson A, et al. . Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75 10.1001/jamaneurol.2018.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2018-320071supp002.pdf (185.4KB, pdf)
jnnp-2018-320071supp001.pdf (41KB, pdf)
jnnp-2018-320071supp003.wmv (25.5MB, wmv)
jnnp-2018-320071supp004.wmv (38.7MB, wmv)
jnnp-2018-320071supp005.wmv (43.9MB, wmv)