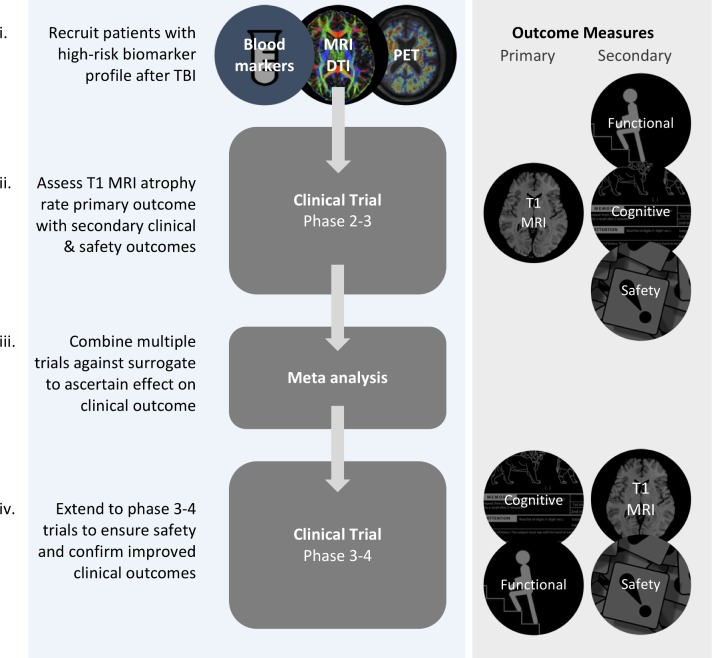
Figure 6.
Biomarkers in clinical trials after traumatic brain injury (TBI). Stages for the evaluation of disease-modifying/neuroprotective treatment after TBI. (i) Recruitment of patients at high risk for neurodegeneration using baseline blood neurofilament light, diffusion tensor imaging abnormality (DTI) and positron emission tomography (PET) abnormality. (ii) Phase 2–3 trials powered to primary outcome measure of change in atrophy rate (using repeated T1 MRI) with secondary functional/cognitive/safety outcomes. (iii) Meta-analysis of phase 2–3 trials to clarify the relationship between the surrogate (T1 atrophy rate) and patient-centred outcomes. (iv) Late-stage phase 3–4 trials using primary functional or cognitive outcome. This may be a composite measure.