Abstract
Introduction
While there is substantial evidence for the benefits of exercise-based rehabilitation in the prevention and management of non-communicable disease (NCD) in high-resource settings, it is not evident that these programmes can be effectively implemented in a low-resource setting (LRS). Correspondingly, it is unclear if similar benefits can be obtained. The objective of this scoping review was to summarise existing studies evaluating exercise-based rehabilitation, rehabilitation intervention characteristics and outcomes conducted in an LRS for patients with one (or more) of the major NCDs.
Methods
The following databases were searched from inception until October 2018: PubMed/Medline, Embase, CINAHL, Cochrane Library, PsycINFO and trial registries. Studies on exercise-based rehabilitation for patients with cardiovascular disease, diabetes, cancer or chronic respiratory disease conducted in an LRS were included. Data were extracted with respect to study design (eg, type, patient sample, context), rehabilitation characteristics (eg, delivery model, programme adaptations) and included outcome measures.
Results
The search yielded 5930 unique citations of which 60 unique studies were included. Study populations included patients with cardiovascular disease (48.3%), diabetes (28.3%), respiratory disease (21.7%) and cancer (1.7%). Adaptations included transition to predominant patient-driven home-based rehabilitation, training of non-conventional health workers, integration of rehabilitation in community health centres, or triage based on contextual or patient factors. Uptake of adapted rehabilitation models was 54%, retention 78% and adherence 89%. The majority of the outcome measures included were related to body function (65.7%).
Conclusions
The scope of evidence suggests that adapted exercise-based rehabilitation programmes can be implemented in LRS. However, this scope of evidence originated largely from lower middle-income, urban settings and has mostly been conducted in an academic context which may hamper extrapolation of evidence to other LRS. Cost-benefits, impact on activity limitations and participation restrictions, and subsequent mortality and morbidity are grossly understudied.
Keywords: noncommunicable disease, rehabilitation, developing countries
Key questions.
What is already known?
The burden of non-communicable disease (NCD) is increasing exponentially in low-resource settings (LRS), where health systems are least equipped to manage these conditions.
Exercise-based rehabilitation is an evidence-based, essential component in the management of NCDs.
What are the new findings?
Sixty studies were identified that evaluated exercise-based rehabilitation, predominantly in urban LRS, and embedded in an academic context.
Research on exercise-based rehabilitation for patients with cancer in LRS is in its infancy.
The majority of outcomes included in these studies pertained to the level of body function (eg, blood pressure), whereas outcomes on the level of activity and participation were reported less, and specific outcomes relevant for policy and guideline development were scarce.
What do the new findings imply?
Important evidence gaps exist in terms of the type of LRS in which exercise-based rehabilitation for NCD has been studied, as well as the specific outcomes included in these studies.
Feasibility of using alternative delivery models to circumvent resource restrictions encourages a wider implementation and study of exercise-based rehabilitation for NCDs in LRS.
Introduction
While there is substantial evidence for the benefits of exercise-based rehabilitation in high-resource settings,1–4 it is not evident that these evidence-based programmes can be implemented in a low-resource setting (LRS), and correspondingly it is not clear whether the same outcomes can be achieved. For example, how is exercise provided effectively in settings where exercise facilities are limited, and patient safety during outdoor exercise is not guaranteed? Moreover, how can a benefit be established using gold-standard methods when, for instance, randomisation to usual care is ethically questionable based on the established evidence of benefit from high-resource settings? Are different outcomes more relevant to patients with non-communicable diseases (NCD) living in an LRS, yet scantly considered in high-resource settings?
NCDs are the leading cause of death globally; almost three-quarters of NCD-related deaths occur in low and middle-income countries (LMIC).5 As of 2017, cardiovascular disease (CVD) accounts for most of these deaths (17.8 million people annually) globally, followed by neoplasms (9.56 million), respiratory diseases (3.91 million) and diabetes (1.37 million).6 These four conditions account for 82% of all NCD deaths and for 54% of loss in disability-adjusted life years (DALY) globally. The burden of NCDs is growing; the WHO projected that by 2030, NCDs in LMICs will be responsible for three times as many DALYs and nearly five times as many deaths compared with deaths caused by communicable diseases, maternal, perinatal and nutritional conditions combined.7 The increasing burden of NCDs is likely to burden health systems least equipped to tackle the challenge,8 and likely to impede poverty reduction initiatives.7 9 While nationwide policies for NCD risk factors such as tobacco use, alcohol and nutrition exist (less so for physical activity), these policies have rarely been adequately implemented due a multitude of factors including inadequate political commitment and resources.8 Rehabilitation is an evidence-based intervention for the management of NCDs,2 10 for which the current need far exceeds the availability, particularly in LRS.11–15
As outlined in the WHO call for action (#REHAB2030), there is a dearth of evidence from randomised controlled trials (RCT) evaluating the effects of rehabilitation in LMICs.7 Understanding the contextual factors which have been reported when investigating exercise-based rehabilitation programmes for the management of NCDs in LRS could inform the planning of future RCTs in this context. Therefore, the objective of this scoping systematic review was to summarise and analyse existing studies evaluating exercise-based rehabilitation, in terms of quality of evidence/methodology, rehabilitation intervention characteristics and outcomes conducted in an LRS for patients with one (or more) of the major NCDs.
Methods
Given the comprehensive nature of this inquiry, a scoping review was conducted based on the model described by Arksey and O’Malley,16 and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis for Scoping Reviews (PRISMA-ScR).17 A PROSPERO-based review registration template is publicly available on the Open Science Framework (https://osf.io/5hu3w/).
Inclusion criteria
The focus of this review was on rehabilitation for patients with NCDs of lifestyle conducted in an LRS. Inclusion and exclusion criteria were revised iteratively based on increasing familiarity with the literature, and related to the participants, concepts and context. All original research study designs reported in English were considered, excluding case studies.
Participants
The four major NCDs related to lifestyle considered were: CVD (International Classification of Diseases (ICD): I0–99), malignant neoplasms (ICD: C00–97), chronic respiratory disease (ICD: J30–98) or diabetes (ICD: E10–E14; excluding those with complications (minus E10.2–E10.29, E11.2–E11.29, E12.2, E13.2–E13.29, E14.2)).18 We also considered studies in which the study sample had at least one of these medical conditions in co-occurrence with other medical conditions due to the high prevalence of multiple medical conditions in LRS (eg, HIV/AIDS).19
Concepts
Rehabilitation was defined in line with the WHO as ‘a set of measures that assist individuals who experience, or are likely to experience, disability to achieve and maintain optimal functioning in interaction with their environments’.7 To be included, the rehabilitation delivered in the studies had to consist of the following: (1) assessment of NCD risk factors, (2) structured exercise (supervised or unsupervised), and (3) at least one additional strategy to control risk factors (eg, education).20 This further specification of rehabilitation was warranted due to the common risk factors (eg, physical inactivity, poor nutrition) associated with the incidence and burden of the four major NCDs studied.
Context
Studies were considered to be conducted in an LRS if they were undertaken in a low-income country (LIC) to lower middle-income country (LM) as per World Bank criteria (83 of 218 countries),21 or in an upper middle-income country (UM) or high-income country (HIC) yet explicitly in a context indicative for an LRS (eg, rural areas, minority populations).
Data sources and search strategy
Six electronic databases were searched on 12 October 2018 and without date limitations: CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane Central Register of Controlled Trials, Embase, Medline (Ovid), PsycINFO and PubMed (excluding Medline records). The search strategies were developed in collaboration with an information specialist (MP), the inclusion of subject headings as appropriate for each database and free-text terms relevant to each key concept. The search results were limited to humans and no language limits were applied. Reference lists of included studies were hand searched for additional materials. The full Medline search strategy is included as online supplementary file 1.
bmjgh-2019-001833supp001.pdf (180.8KB, pdf)
Study selection
A rough initial screening of titles was conducted by one reviewer (MH), and titles clearly ineligible were excluded at this stage. Subsequently, potentially eligible titles and abstracts were screened independently by two reviewers (MH and ALS) to determine the inclusion or exclusion of studies for full-text review. Any disagreements were discussed and used to refine inclusion and exclusion criteria. In case of irreconcilable disagreements, a third reviewer was consulted (SDH). An identical process was used to determine final inclusion of full-text articles obtained. The citation inclusion and exclusion consideration process and data extraction were performed within an online review management platform (https://www.covidence.org).
Data extraction and synthesis
A charting form was developed to characterise the thematic focus of each paper, study design (eg, type, patient sample, geographical context, setting, outcome measures), rehabilitation intervention characteristics (eg, delivery model, risk factors addressed, exercise prescription), deliberate choices made to accommodate the LRS, utilisation (uptake (eligibility vs inclusion), loss to follow-up and adherence), as well as qualitative and quantitative results. Included articles were equally allocated among two reviewers (MH and ALS). The first reviewer performed the data extraction, while the second reviewer verified the data extracted and vice versa (as opposed to extracting all data independently). This process was considered a pragmatic way to share the workload while maintaining scientific rigour. An exception to this process was the risk of bias of the included clinical trials, based on the Cochrane Risk of Bias tool, which was conducted by two reviewers (MH and ALS) independently.22 Studies were considered of high quality when meeting all criteria (ie, low risk of bias) except blinding of participants, which was deemed unfeasible in most rehabilitation-type interventions.23 The extraction form was developed a priori, and revised after each included study until no further changes to the form were considered necessary.
Reported outcomes for each study were extracted and pooled according to the WHO International Classification of Functioning, Disability and Health (ICF) model.24 The ICF model describes the relation and interaction between the health condition, impairments of body function and structures (eg, lung function, lipid profile), activity limitations (eg, mobility, balance) and participation restrictions (eg, productivity, quality of life), personal factors (eg, stress, anxiety, depression) and environmental factors (eg, social support, access to technology). The ICF model is the WHO preferred framework for the assessment of health and disability at an individual and population level.24 Grouping the outcomes under specific ICF brackets was done by two authors independently (MH and ALS), and discussed in the case of disagreement. A narrative qualitative synthesis of results was undertaken for all included studies and supported by descriptive statistics of metadata. A secondary analysis, not a priori specified, was included to determine if specific outcome types are assessed in different settings using a χ2 test for categorical data.
Patient and public involvement
Patient or public were not involved in the design, conduct or reporting of this scoping review.
Results
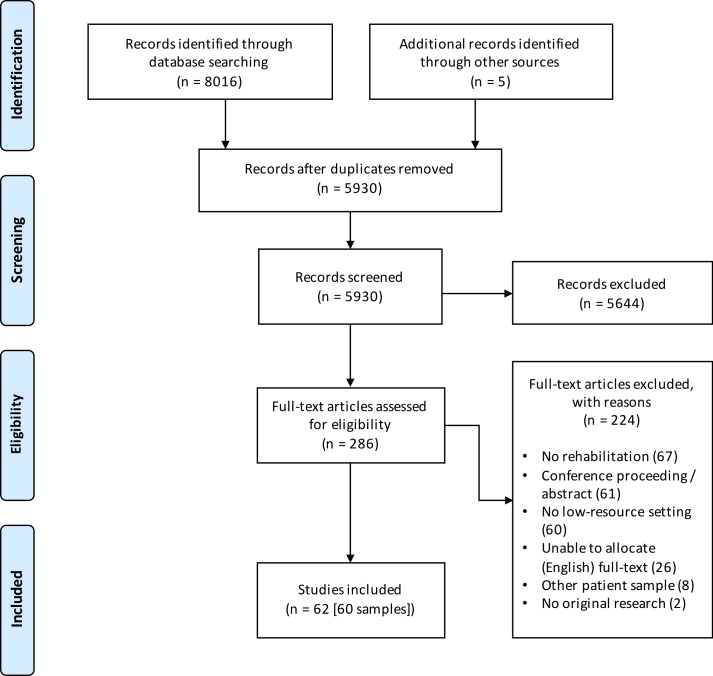
See figure 1 for a PRISMA flow chart. Of the 8021 records identified, 5930 remained after elimination of duplicates. An initial screening of titles in stage I and title/abstracts in stage II (n=1229) reduced this number to 286. The full-text versions of the remaining articles were reviewed for eligibility resulting in the exclusion of 224 for the reasons noted. Ultimately, 62 studies were included from the search, reporting on 60 unique patient samples.23 25–86
Figure 1.
Study selection process.
Disease profile and geographical characteristics
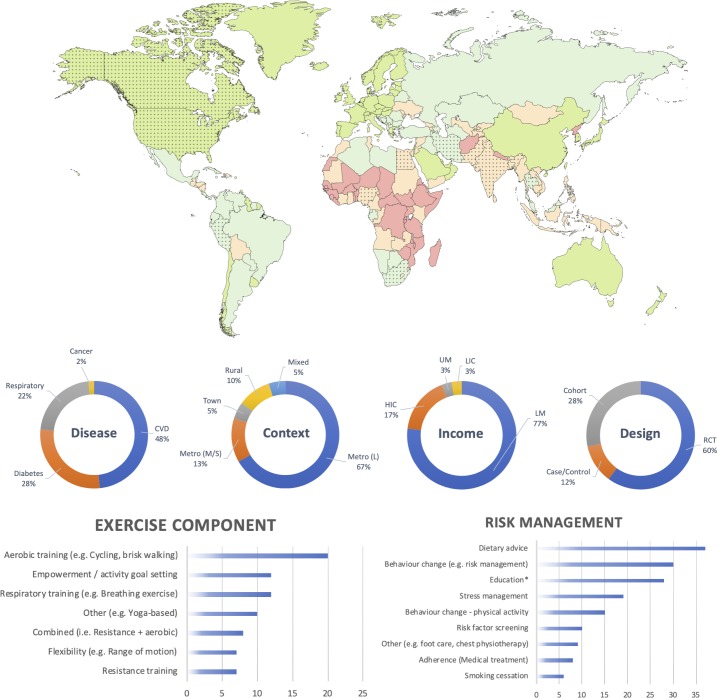
Figure 2 provides a graphical synthesis of the study characteristics including disease profile, geographical context, income classification, study design, exercise and risk management components included in the sample. A full table with all individual studies, their characteristics and rehabilitation programmes can be found in the online supplementary file 2. Twenty-nine studies included patients with CVD (48.3%), 17 studies (28.3%) included patients with diabetes, 13 studies (21.7%) involved patients with respiratory disease and a single study (1.7%) was found on patients with cancer. Studies were predominantly conducted in large metropolitan cities (n=40 (66.7%)) of LMs (n=46 (76.7%)). Two studies (3.3%) were identified in an LIC (both India) at the time of the conduct of the study (India later promoted to LM). In addition to studies in LICs and LMs, a proportion of studies were conducted in a low-resource context of either a UM (n=2 (3.3%)), or in an HIC (n=10 (16.7%)). The 10 studies in HICs targeted underserved or minority populations living in a poor socioeconomic context. Countries that were often represented included India (n=22 (36.7%)), Egypt (n=12 (20.0%)) and the USA (n=7 (11.7%)). Few studies were undertaken in sub-Saharan Africa (n=3 (5.0%)) and on the South American continent (n=1 (1.7%)). Thirty-seven (61.7%) studies were conducted in an academic setting or academic capacity (eg, university hospital, research providing intervention). A majority of studies (n=36 (60.0%)) did not receive or report funding; where studies were funded, most had national/government funding (n=19 (31.7%)), with fewer having international funding (n=3 (5.0%)), and 2 (3.4%) being self-funded.
Figure 2.
Graphical synthesis of results extracted from online supplementary file 2 (characteristics of included studies). Top: the world map of countries per 2016 World Bank income classification (low-income country (LIC), red; lower middle-income country (LM), orange; upper middle-income country (UM), light green; high-income country (HIC), dark green). Dotted countries are countries in which one or more studies were undertaken. Middle: percentage (%) of studies per disease group, geographical context, income classification and study design. Bottom left: number of studies that included a specific exercise component. Bottom right: number of studies that included specific other methods to control for risk factors. *Education pertains to, for instance, self-management, or knowledge of underlying pathology. CVD, cardiovascular disease; Metro, Metropolitan (S, Small; M, Medium; L, Large); RCT, randomised controlled trial.
bmjgh-2019-001833supp002.pdf (457.8KB, pdf)
Methodological characteristics
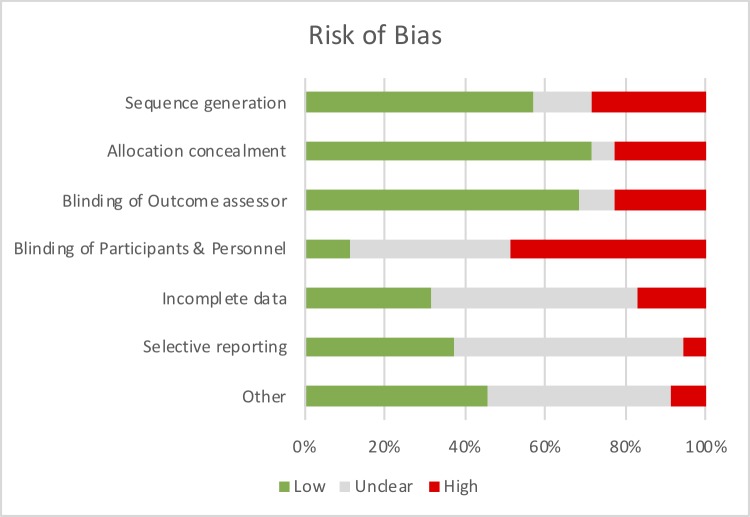
Seven (11.7%) of the studies used a case–control design, of which six were prospective (mean±SD=73.8±35.9 participants/study) and one retrospective (30 participants). Seventeen (28.3%) cohort studies were included, of which 15 were prospective (mean±SD=116.6±146.6 participants/study), and two were retrospective in design (mean±SD=463.5±598.9 participants/study). The remaining 36 studies (60.0%) were RCTs (mean±SD=94.0±80.7 participants/study). Nine RCTs (25.0%) reported a clinical trial registration. Fifteen (41.7%) reported a sample size calculation, of which nine achieved the calculated sample size (60.0%). Two studies (5.6%) were of high methodological quality (figure 3; online supplementary file 4). Risk of bias was found predominantly in the randomisation and allocation concealment (or reporting thereof). Two studies (3.4%) reported specific considerations in terms of the research methodology (eg, randomisation at study site level to enable focused allocation of resource, for instance,76 in the light of a rural context where individuals are geographically far apart).
Figure 3.
Risk of bias of included randomised controlled trials (RCT) (n=36); see online supplementary file 4 for judgement per individual study.
bmjgh-2019-001833supp004.pdf (200.4KB, pdf)
Rehabilitation model and model adaptations
Online supplementary file 2 provides a per-study overview of the rehabilitation interventions delivered, their primary exercise component (eg, walking) and methods to manage risk factors (eg, smoking cessation). Figure 2 provides a graphical synthesis of online supplementary file 2. Table 1 provides a quantitative synthesis of the intervention delivery model (eg, outpatient). In most cases, rehabilitation was delivered on an outpatient (n=25 (41.7%)) or ‘hybrid’ (n=17 (28.3%); that is, supervised transitioning to unsupervised setting) basis. Most programmes comprised exercise and education (n=37 (61.7%)). Most rehabilitation was delivered by a variety of healthcare professionals including physiotherapists or community health workers (n=38 (63.4%); see online supplementary file 2 for details regarding the types of healthcare professionals). The mean duration of programmes was 18 weeks (SD=21; range=0.7–104.0); programmes offered a mean of 27 supervised sessions (SD=40; range 0–224) of 75 min in duration on average (SD=56; range=4–360). The nature of control groups differed across the studies. Eleven out of 36 RCTs (27.8%) had an active comparison group (eg, different exercise paradigm), 11 (30.6%) had a usual care control, 6 (16.7%) usual care plus a low-intensity add-on, 6 (16.7%) reported ‘no intervention’ for the control group, and for 2 (5.6%) studies it was not reported. Detailed reporting on the nature of usual care was often limited. However, based on 32 (94% of RCTs) studies, usual care generally consisted of pharmacological management plus an add-on component to control for attention (eg, dietary consultation (n=9, 26.5%)), pharmacological management only (n=6; 17.6%), education only (n=5; 14.7%), no management (n=4; 11.8%), medical management (n=4; 11.8%), conventional rehabilitation (n=3; 8.9%) or other (n=1; 2.9%).
Table 1.
Rehabilitation intervention characteristics by country income classification
| Setting | Number of studies (n=60) | Total (%) | Per World Bank income classification (%) | |||
| LIC (n=2) | LM (n=46) | UM (n=2) | HIC (n=10) | |||
| Inpatient | 3 | 5.0 | 0.0 | 7.1 | 0.0 | 0.0 |
| Outpatient | 24 | 40.0 | 0.0 | 52.4 | 0.0 | 0.0 |
| Community | 8 | 13.3 | 100.0 | 6.5 | 50.0 | 40.0 |
| Home based | 8 | 13.3 | 0.0 | 4.8 | 0.0 | 40.0 |
| Hybrid | 17 | 28.3 | 0.0 | 30.4 | 50.0 | 20.0 |
| Inpatient and home based | 5 | 29.4 | 0.0 | 23.1 | 0.0 | 50.0 |
| Outpatient and home based | 4 | 23.5 | 0.0 | 23.1 | 0.0 | 50.0 |
| Inpatient and outpatient | 4 | 23.5 | 0.0 | 30.8 | 0.0 | 0.0 |
| Inpatient, outpatient and home based | 3 | 17.6 | 0.0 | 23.1 | 0.0 | 0.0 |
| Community and home based | 1 | 5.9 | 0.0 | 0.0 | 100.0 | 0.0 |
| Intervention type | ||||||
| Primarily exercise | 12 | 20.0 | 0.0 | 26.2 | 0.0 | 0.0 |
| Primarily education | 12 | 20.0 | 0.0 | 8.7 | 50.0 | 70.0 |
| Primarily exercise and education | 36 | 60.0 | 100.0 | 63.0 | 50.0 | 30.0 |
| Primary person responsible | ||||||
| Healthcare professional (HCP) | 37 | 61.7 | 0.0 | 58.7 | 50.0 | 60.0 |
| Patient | 15 | 25.0 | 100.0 | 21.7 | 50.0 | 30.0 |
| Equal between HCP and patient | 2 | 3.3 | 0.0 | 4.8 | 0.0 | 0.0 |
| Unspecified | 6 | 10.0 | 0.0 | 11.9 | 0.0 | 10.0 |
HIC, high-income country; LIC, low-income country; LM, lower middle-income country; UM, upper middle-income country.
Table 2 provides an overview of strategies to optimise the methodologies and intervention characteristics to accommodate contextual restraints. These strategies were either explicitly mentioned by the authors or were independently (MH and ALS) identified adaptations during data extraction. The most commonly reported strategies to accommodate such a setting were the adoption of alternative delivery models (eg, text messaging, home-based models, use of ‘non-conventional’ healthcare workers) and incorporating cultural aspects into the design of the intervention (eg, introducing yoga as an exercise component).
Table 2.
Considerations for rehabilitation in an LRS implemented in included studies
| Considerations for LRS | n | % |
| Home-based programmes | 7 | 11.5 |
| Adapting programmes to patients’ cultural background | 7 | 11.5 |
| Adapting programmes to resources available | 6 | 9.8 |
| Tailoring of educational material | 5 | 8.2 |
| Simple language (low literacy) | 2 | 40.0 |
| Culturally appropriate information | 2 | 40.0 |
| Graphics (low literacy) | 1 | 20.0 |
| Inclusion of family members | 5 | 8.2 |
| Outreach (medical team travel to community and home visits) | 3 | 4.9 |
| Peer accountability through peer groups | 2 | 3.3 |
| Adaptations to study design | 2 | 3.3 |
| Initiating rehabilitation in hospital before discharge | 1 | 1.6 |
| Use of technology (eg, smartphones) | 1 | 1.6 |
| Active exclusion due to accessibility | 1 | 1.6 |
LRS, low-resource setting; n, number of times reported.
Outcomes
A total of 432 outcomes were extracted across the 60 studies (see online supplementary file 3); these were grouped according to the ICF24 into outcomes relating to body function and impairments (n=284 (65.7%)), activity limitations and participation restrictions (n=120 (27.8%)), personal factors (n=26 (6.0%)) or environmental factors (n=2 (0.1%)). Outcome measures that were reported most often included the 6 min walk test (n=20), body mass index (n=19) and blood pressure (n=17). There was no significant difference in the types of outcomes measured based on World Bank income classifications or study design. However, there was a significant difference in outcome types measured by NCD (χ2=39.7 (p<0.01)). A higher proportion of studies in patients with respiratory disease assessing outcomes related to activity limitations and participation restrictions compared with CVD and diabetes.
bmjgh-2019-001833supp003.pdf (280.8KB, pdf)
Four studies (6.7%) reported specific feasibility outcomes (eg, participation rates, referral rates, hospitalisation) in relation to their study objective(s). When considering the included RCTs, uptake was on average (SD; number of studies) 54% (SD 32%; n=17), retention was 78% (SD 31%; n=35) and adherence to the experimental intervention 89% (SD 12%; n=5).
Discussion
Through this review, 60 studies were identified across four key contributors to the burden of NCDs. Alternative delivery models tested in the LRS were practical in circumventing resource-related barriers as indicated by the retention and adherence reported. Among others, these delivery models included transition to predominantly patient-driven, home-based rehabilitation, training of non-medical or non-conventional health workers (eg, community or auxiliary health workers, nurses), integration of rehabilitation in community (primary) health centres, or triage patients based on contextual factors (eg, distance to centre) or medical profile (eg, risk stratification, comorbidity profile). However, the methodological quality of the included studies was low, and often lacked outcomes that can drive policy and guideline development. Surprisingly, only a single study on the exercise-based rehabilitation of patients with cancer in an LRS was found, indicating that this particular field is still in its infancy. In addition, if we consider the increasing burden of NCDs in LRS, the small body of evidence for exercise-based rehabilitation in South America and (sub-Saharan) Africa particularly is concerning. In addition to the evidence that was not found, three important gaps could be derived from the research included in this review.
First, while a number of studies conveyed the need for low-cost or cost-effective models of rehabilitation to address the increasing burden of NCD in LRS, the studies in this review failed to include economic measures in their evaluations. In line with this, there was a paucity of studies reporting mortality and morbidity outcomes, which are typically used in health economic analyses to calculate DALYs. Also, none of the included studies administered the EuroQol-5 Dimension, a common outcome measure of health-related quality of life used for quantifying quality-adjusted life years (QALY). Unfortunately, the lack of these health economic measures (DALYs, QALYs) may hamper the incorporation of rehabilitation into policy guidelines and initiatives (eg, national health insurance). A synthesis of 19 studies on the cost-effectiveness of cardiac rehabilitation, all in UM or HIC, suggested that cardiac rehabilitation is cost-effective, especially with exercise as a component.87 There may be a myriad of reasons as to why health economic analyses are absent in LRS. First, there may be limited academic capacity in LRS to conduct and advise on health economic analyses as this is a relative new field within rehabilitation medicine. Second, the scarcity, quality and accessibility of data often attributable to the absence of financial cost registration and limited patient information systems to obtain rigorous data in morbidity and mortality, may impose significant challenges in the conduct of economical evaluations in LRS. A way forward may be to contest the notion that the cost-benefits of rehabilitation for NCDs in LRS should be expressed in relation to morbidity and mortality. Other indicators including socioeconomic parameters, equity measures, societal participation or food security may be considered more applicable alternatives. The other indicators may be more easily accessible to express the benefits relative to the cost, for both the provider as well as the direct (eg, out-of-pocket expenses for exercise equipment) and indirect (eg, transport) costs to the patient. With respect to the latter, only few studies (n=2) were identified in LICs where low cost-to-patient models are essential to prevent patients from falling into a medical poverty trap.88
Second, most studies included outcome measures related to body function and impairment whereas few studies reported (primary) outcomes on the level of activity (eg, mobility, physical activity) or participation (eg, productivity). While these physiological outcomes are essential in terms of reducing mortality, morbidity and, therefore, drive health policy, one may postulate that outcomes on the level of activity limitations and participation restrictions may be particularly of interest in low-resource environments, and more relevant to the field of rehabilitation medicine. With the average age for patients with NCD in LRS versus HIC being ~10 years younger,89 reporting on outcomes that measure participation restrictions could highlight the potential value of physical rehabilitation as an intervention. As patients suffering from NCD could still actively provide an active economic contribution, demonstrating the impact of rehabilitation on limiting productivity years lost may accelerate initiatives to increase access to rehabilitation in LRS.
Finally, there are few studies that detail the effect of alternative delivery models on access and uptake of rehabilitation interventions for NCDs. This is in addition to other potential external mechanisms that may increase uptake (eg, clinician education, financial incentives).90 The uptake of rehabilitation interventions reported in this review ranged substantially (11%–100%) and hampered by the large proportion of studies that was embedded in an academic setting. Going forward, it is therefore important that future studies in this field operate outside of the academic setting to increase the knowledge transfer and implementation from research to community, and better understand the impact of a particular delivery model on uptake and adherence measures.91 In the light of universal health coverage, and upscaling rehabilitation access in general, understanding contextual knowledge translation is an essential step in addressing the dearth of evidence on rehabilitation for NCDs in LRS as identified in the REHAB2030 call for action.
There are a number of limitations to this review. First, the data sources search for this review has been restricted to major databases (eg, Medline) and literature published in English. Subsequently, the 62 reports included in this review may not be entirely inclusive for the entire scope of work done. The absence of the Latin American and Caribbean Health Sciences Literature (LILACS) and Africa Wide literature database, for instance, may have resulted in a failure to identify studies specifically in the South American and African continents. However, despite the variety of populations included in this review (eg, diabetes, CVD), similar ‘themes’ emerged from these studies. Hence, the 62 studies included in this review provide a compelling overview of the scope of evidence.
Second, the concept of rehabilitation is ambiguous and there are a variety of definitions and models. This often involves individualised, person-centred elements that defy easy standardisation.92 This is also reflected in the broad search strategy used, and consequently the high number of exclusions/ineligible articles found. A sensible operationalisation of what constitutes rehabilitation may have improved the reliability when judging eligibility for this review. In this specific review, an exercise component was a prerequisite for inclusion as (A) particular rehabilitation models with an exercise component have been shown to be cost-effective,87 and (B) it would strengthen the agreement between reviewers during the inclusion process. While the mandatory inclusion of an exercise component as part of comprehensive rehabilitation is plausible in high-income contexts, we also need to consider that (1) physical inactivity may not always be the primary working mechanism behind the prevalence of NCDs and as such, this inclusion criterion may have resulted in a failure to identify studies relevant to this review,93 and (2) specifically in the context of alternative delivery models, even the definition of what constitutes exercise training (or rehabilitation in general) needs to be broadened.
A third construct that is ambiguous is that of a ‘low-resource setting’. Studies and reviews with a similar scope often opt to consider either LMIC (ie, World Bank classification) or developing countries (eg, Gini index) as more objective constructs to classify countries or study context. However, various studies in this review confirm that access and uptake of rehabilitation in a resource-constrained context is not limited to LMICs; challenging and complex circumstances are found both in HIC and UM as well. Conversely, also high-resource programmes are found in LMs. Hence, the definitions chosen in this review to some extent drive the generalisability of the results.
Conclusions
Various innovative considerations to address contextual factors of providing exercise-based rehabilitation within an LRS have been reported. In addition, the review highlights the paucity of data available on the potential impact of exercise-based rehabilitation on key policy drivers including mortality and morbidity, cost-benefits and outcomes on a participation level. The information summarised in this review can now be used by researchers in the planning of high-quality experimental studies to address the evidence deficit for exercise-based rehabilitation in the management of NCDs in LRS.
Footnotes
Handling editor: Seye Abimbola
Twitter: @m_heine01, @sherrylgrace
Contributors: MH conceived the review. All authors (MH, ALS, MP, SG, WD, SDH) developed the methods. MH and ALS had primary responsibility for the analysis and initial draft of the manuscript. All authors contributed substantially to the analysis, interpretation of the results and completion of the manuscript. All authors approved the final manuscript.
Funding: This study is funded by the AXA Research Fund (S005459).
Disclaimer: The funder (AXA Research Fund) of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Anderson L, Thompson DR, Oldridge N, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long L, Anderson L, Dewhirst AM, et al. . Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev 2018;2 10.1002/14651858.CD012786.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puhan MA, Gimeno‐Santos E, Cates CJ, et al. . Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library. John Wiley & Sons, Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris NR, Kermeen FD, Holland AE. Exercise‐based rehabilitation programmes for pulmonary hypertension. In: The Cochrane Library. John Wiley & Sons, Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Global status report on noncommunicable diseases, 2014. Available: http://www.who.int/nmh/publications/ncd-status-report-2014/en/ [Accessed 25 Jun 2018].
- 6.Roth GA, Abate D, Abate KH, et al. . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO | Rehabilitation A Call for Action. WHO, 2030. Available: http://www.who.int/rehabilitation/rehab-2030/en/ [Accessed 22 Mar 2018].
- 8.Juma PA, Mohamed SF, Matanje Mwagomba BL, et al. . Non-Communicable disease prevention policy process in five African countries authors. BMC Public Health 2018;18:961 10.1186/s12889-018-5825-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owolabi M, Miranda JJ, Yaria J, et al. . Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Glob Health 2016;1:e000105 10.1136/bmjgh-2016-000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long L, Mordi IR, Bridges C, et al. . Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;8 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesah E, Turk-Adawi K, Supervia M, et al. . Cardiac rehabilitation delivery in low/middle-income countries. Heart 2019. doi: 10.1136/heartjnl-2018-314486. [Epub ahead of print: 28 Jun 2019]. [DOI] [PubMed] [Google Scholar]
- 12.Heine M, Turk-Adawi K, Supervia M, et al. . Cardiac rehabilitation delivery in Africa. Cardiovasc J Afr 2019;30:133–7. 10.5830/CVJA-2019-011 [DOI] [PubMed] [Google Scholar]
- 13.Jesus T, Landry M, Hoenig H. Global need for physical rehabilitation: systematic analysis from the global burden of disease study 2017. Int J Environ Res Public Health;16:980 10.3390/ijerph16060980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bright T, Wallace S. Kuper H. a systematic review of access to rehabilitation for people with disabilities in low- and middle-income countries. Int J Environ Res Public Health;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamenov K, Mills J-A, Chatterji S, et al. . Needs and unmet needs for rehabilitation services: a scoping review. Disabil Rehabil 2019;41:1227–37. 10.1080/09638288.2017.1422036 [DOI] [PubMed] [Google Scholar]
- 16.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, et al. . PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med (Epub ahead of print 4 September 2018). [DOI] [PubMed] [Google Scholar]
- 18.WHO who methods and data sources for global burden of disease estimates 2000-2011. Geneva: Dep Health Stat Inf Syst. [Google Scholar]
- 19.Wyk P-van, Msemburi W, Laubscher R, et al. . Mortality trends and differentials in South Africa from 1997 to 2012: second National burden of disease study. Lancet Glob Health 2016;4:e642–53. [DOI] [PubMed] [Google Scholar]
- 20.Grace SL, Turk-Adawi KI, Contractor A, et al. . Cardiac rehabilitation delivery model for low-resource settings. Heart 2016;102:1449–55. 10.1136/heartjnl-2015-309209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank Country and Lending Groups – world bank data help desk. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Accessed 4 September 2018].
- 22.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: http://handbook.cochrane.org
- 23.Jayasheela H, Sivabalan T. Effect of pulmonary rehabilitation on quality of life among the chronic obstructive disease patients admitted at Pravara rural Hospital, Loni (BK). Inter. Jour. of Nurs. Educ. 2017;9:102–7. 10.5958/0974-9357.2017.00045.9 [DOI] [Google Scholar]
- 24.WHO International Classification of Functioning, Disability and Health (ICF). WHO. Available: http://www.who.int/classifications/icf/en/ [Accessed 25 September 2018].
- 25.Abdelhalem AM, Shabana AM, Onsy AM, et al. . High intensity interval training exercise as a novel protocol for cardiac rehabilitation program in ischemic Egyptian patients with mild left ventricular dysfunction. Egypt Heart J 2018;70:287–94. 10.1016/j.ehj.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajiboye OA, Anigbogu CN, Ajuluchukwu JN, et al. . Exercise training improves functional walking capacity and activity level of Nigerians with chronic biventricular heart failure. Hong Kong Physiotherapy Journal 2015;33:42–9. 10.1016/j.hkpj.2014.11.002 [DOI] [Google Scholar]
- 27.Ali MS, Talwar D, Jain SK. The effect of a short-term pulmonary rehabilitation on exercise capacity and quality of life in patients hospitalised with acute exacerbation of chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2014;56:13–19. [PubMed] [Google Scholar]
- 28.Babu A, Noone M, Haneef M, et al. . Protocol-guided phase-1 cardiac rehabilitation in patients with ST-elevation myocardial infarction in a rural hospital. Heart Views 2010;11:52–6. 10.4103/1995-705X.73209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babu A, George MMilton, Guddattu V, et al. . Effects of combined early in-patient cardiac rehabilitation and structured home-based program on function among patients with congestive heart failure: a randomized ontrolled trial. Heart Views 2011;12:99–103. 10.4103/1995-705X.95064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basri R, Tahir M, Naseem M. Short-Term effects of chest physiotherapy in acute exacerbation of chronic obstructive pulmonary disease. J Med Sci 2017;25:323–7. [Google Scholar]
- 31.Biswas R, Bhattacharya M, Biswas A, et al. . The effect of exercises with relaxation technique on quality of life improvements in cancer haematology patients following chemotherapy. Indian J Physiother Occup Ther 2017;11:120–4. [Google Scholar]
- 32.Chakraborty K, Das M, Mandal P, et al. . A comparative study on the effects of comprehensive rehabilitation in uncomplicated coronary artery bypass grafting patients from rural and urban India. Indian J Phys Med Rehabil 2007;18:34–40. [Google Scholar]
- 33.Chi-Jane W, Fetzer SJ, Yi-Ching Y, et al. . The efficacy of using self-monitoring diaries in a weight loss program for chronically obese adults in a rural area. J Nurs Res Lippincott Williams Wilkins 2012;20:181–8. [DOI] [PubMed] [Google Scholar]
- 34.Chockalingam P, Sakthi Vinayagam N, Ezhil Vani N, et al. . Outcomes of a multidisciplinary coronary heart disease prevention programme in southern India. Heart Asia 2016;8:39–44. 10.1136/heartasia-2016-010791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung B, Kim D, Nam EW. Evaluation of hypertension prevention and control programs in Lima, Peru. Osong Public Health Res Perspect 2018;9:36–41. 10.24171/j.phrp.2018.9.1.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daabis R, Hassan M, Zidan M. Endurance and strength training in pulmonary rehabilitation for COPD patients. Egyptian Journal of Chest Diseases and Tuberculosis 2017;66:231–6. 10.1016/j.ejcdt.2016.07.003 [DOI] [Google Scholar]
- 37.Davis TC, Seligman HK, DeWalt DA, et al. . Diabetes implementation of a self-management program in resource poor and rural community clinics. J Prim Care Community Health 2012;3:239–42. 10.1177/2150131911435673 [DOI] [PubMed] [Google Scholar]
- 38.Dehdari T, Heidarnia A, Ramezankhani A, et al. . Effects of progressive muscular relaxation training on quality of life in anxious patients after coronary artery bypass graft surgery. Indian J Med Res 2009;129:603–8. [PubMed] [Google Scholar]
- 39.Dhameja K, Singh S, Mustafa MD, et al. . Therapeutic Effect of Yoga in Patients with Hypertension with Reference to GST Gene Polymorphism. The Journal of Alternative and Complementary Medicine 2013;19:243–9. 10.1089/acm.2011.0908 [DOI] [PubMed] [Google Scholar]
- 40.Edla SR, Kumar AMV, Srinivas B, et al. . ‘Integrated Naturopathy and Yoga’ reduces blood pressure and the need for medications among a cohort of hypertensive patients in South India: 3-months follow-up study. Advances in Integrative Medicine 2016;3:90–7. 10.1016/j.aimed.2016.11.001 [DOI] [Google Scholar]
- 41.El Demerdash S, Khorshid H, Salah I, et al. . Cardiac rehabilitation improves the ischemic burden in patients with ischemic heart disease who are not suitable for revascularization. Cardiovascular Revascularization Medicine 2015;16:280–3. 10.1016/j.carrev.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 42.El-Helow MR, Zamzam ML, Fathalla MM, et al. . Efficacy of modified constraint-induced movement therapy in acute stroke. Eur J Phys Rehabil Med 2015;51:371–9. [PubMed] [Google Scholar]
- 43.Elshazly A, Khorshid H, Hanna H, et al. . Effect of exercise training on heart rate recovery in patients post anterior myocardial infarction. Egypt Heart J 2018;70:283–5. 10.1016/j.ehj.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Embarak S, Mansour W, Mortada MA. Pulmonary rehabilitation slows the decline in forced expiratory volume in 1 second and improves body mass index in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc 2015;64:41–5. 10.1016/j.ejcdt.2014.08.009 [DOI] [Google Scholar]
- 45.Eraballi A, Raghuram N, Ramarao NH, et al. . Yoga based lifestyle program in improving quality of life after coronary artery bypass graft surgery: a randomised controlled trial. J Clin Diagn Res 2018;12:YC05–9. [Google Scholar]
- 46.Evans-Hudnall GL, Stanley MA, Clark AN, et al. . Improving secondary stroke self-care among underserved ethnic minority individuals: a randomized clinical trial of a pilot intervention. J Behav Med 2014;37:196–204. 10.1007/s10865-012-9469-2 [DOI] [PubMed] [Google Scholar]
- 47.Ghanem M, ELaal E, Mehany M, et al. . Home-Based pulmonary rehabilitation program: effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann Thorac Med 2010;5:18–25. 10.4103/1817-1737.58955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghashghaei FE, Sadeghi M, Rabiei K, et al. . Gender differences in risk factors of obese patients after cardiac rehabilitation program. Iran J Nurs Midwifery Res 2012;17:381–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Sadeghi M, Ghashghaei FE, Rabiei K, et al. . Is there any difference between non-obese male and female in response to cardiac rehabilitation programs? J Res Med Sci 2012;17:787–91. [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta L, Ghataliya SP, Anand AK, et al. . Effect of yoga training in hypertensive patients. Indian J Appl-Basic Med Sci 2011;13:383–6. [Google Scholar]
- 51.Hassan AM, El Nahas NG. Efficacy of cardiac rehabilitation after percutaneous coronary intervention. Int J PharmTech Res 2016;9:134–41. [Google Scholar]
- 52.Hassan M, Mourad S, Abdel Wahab NH, et al. . Effect of comorbidities on response to pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65:63–9. 10.1016/j.ejcdt.2015.11.006 [DOI] [Google Scholar]
- 53.Jayasuriya R, Pinidiyapathirage MJ, Jayawardena R, et al. . Translational research for diabetes self-management in Sri Lanka: a randomized controlled trial. Prim Care Diabetes 2015;9:338–45. 10.1016/j.pcd.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 54.Jyotsna V, Ambekar S, Singla R, et al. . Cardiac autonomic function in patients with diabetes improves with practice of comprehensive yogic breathing program. Indian J Endocrinol Metab 2013;17:480–5. 10.4103/2230-8210.111645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karimi H, Rehman SSU, Gillani SA. Effects of supervised structured aerobic exercise training program on interleukin-6, nitric oxide synthase-1, and cyclooxygenase-2 in type 2 diabetes mellitus. Jcpsp J Coll Physicians Surg - Pak 2017;27:352–5. [PubMed] [Google Scholar]
- 56.Shakil-Ur-Rehman S, Karimi H, Gillani SA. Effects of supervised structured aerobic exercise training program on fasting blood glucose level, plasma insulin level, glycemic control, and insulin resistance in type 2 diabetes mellitus. Pak J Med Sci 2017;33:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.IS K, Lee TH, Kim GS, et al. . Effects of visiting nurses’ individually tailored education for low-income adult diabetic patients in Korea. Public Health Nurs 2011;28:429–37. [DOI] [PubMed] [Google Scholar]
- 58.Maharaj SS, Nuhu JM. Rebound exercise: a beneficial adjuvant for sedentary non-insulin-dependent type 2 diabetic individuals in a rural environment. Aust J Rural Health 2016;24:123–9. 10.1111/ajr.12223 [DOI] [PubMed] [Google Scholar]
- 59.Masrul DE, Purnakarya I, et al. . Effect of modified diet and exercise on insulin level in diabetes mellitus type-2 patients. Pak J Nutr 2016;15:370–3. [Google Scholar]
- 60.Mayer-Davis EJ, D'Antonio AM, Smith SM, et al. . Pounds off with Empowerment (power): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health 2004;94:1736–42. 10.2105/AJPH.94.10.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehani SHM. Correlation between changes in diastolic dysfunction and health-related quality of life after cardiac rehabilitation program in dilated cardiomyopathy. Journal of Advanced Research 2013;4:189–200. 10.1016/j.jare.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammed HG, Shabana AM. Effect of cardiac rehabilitation on cardiovascular risk factors in chronic heart failure patients. The Egyptian Heart Journal 2018;70:77–82. 10.1016/j.ehj.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moncrieft AE, Llabre MM, McCalla JR, et al. . Effects of a multicomponent life-style intervention on weight, glycemic control, depressive symptoms, and renal function in low-income, minority patients with type 2 diabetes: results of the community approach to lifestyle modification for diabetes randomized controlled trial. Psychosom Med 2016;78:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Najafi F, Nalini M. Hospital-Based versus hybrid cardiac rehabilitation program in coronary bypass surgery patients in Western Iran: effects on exercise capacity, risk factors, psychological factors, and quality of life. J Cardiopulm Rehabil Prev 2015;35:29–36. [DOI] [PubMed] [Google Scholar]
- 65.Nalini M. Outpatient cardiac rehabilitation use after coronary bypass surgery in the West of Iran. J Cardiopulm Rehabil Prev 2014;34:263–70. 10.1097/HCR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 66.Naser A, Shahamfar J, Kumar GV, et al. . Cardiac risk factor changes through an intensive multifactorial life style modification program in CHD patients: results from a two year follow up. J Biol Sci 2008;8:248–57. [Google Scholar]
- 67.Pande A, Singhal P, Kumar R, et al. . Effect of home-based pulmonary rehabilitation programme on disability in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2005;47:217–9. [PubMed] [Google Scholar]
- 68.Raghuram N, Parachuri VR, Swarnagowri MV, et al. . Yoga based cardiac rehabilitation after coronary artery bypass surgery: one-year results on LVEF, lipid profile and psychological states – a randomized controlled study. Indian Heart J 2014;66:490–502. 10.1016/j.ihj.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramirez M, Wu S. Phone messaging to prompt physical activity and social support among low-income Latino patients with type 2 diabetes: a randomized pilot study. JMIR Diabetes 2017;2:e8 10.2196/diabetes.7063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranjita R, Hankey A, Nagendra HR, et al. . Yoga-based pulmonary rehabilitation for the management of dyspnea in coal miners with chronic obstructive pulmonary disease: a randomized controlled trial. J Ayurveda Integr Med 2016;7:158–66. 10.1016/j.jaim.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rifaat N, Anwar E, Ali YM, et al. . Value of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Egyptian Journal of Chest Diseases and Tuberculosis 2014;63:1013–7. 10.1016/j.ejcdt.2014.06.004 [DOI] [Google Scholar]
- 72.Shagufta S, Moiz JA, Aggarwal R. Effect of supervised versus home based cardiac rehabilitation on heart rate recovery in patients with coronary artery bypass grafting. Indian J Physiother Occup Ther 2011;5:199–202. [Google Scholar]
- 73.Shah VO, Carroll C, Mals R, et al. . A home-based educational intervention improves patient activation measures and diabetes health indicators among Zuni Indians. PLoS One;10:e0125820 10.1371/journal.pone.0125820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sindhwani G, Verma A, Biswas D, et al. . A pilot study on domiciliary pulmonary rehabilitation programme in the management of severe chronic obstructive pulmonary disease. Singapore Med J 2011;52:689–93. [PubMed] [Google Scholar]
- 75.Singh V, Khandelwal DC, Khandelwal R, et al. . Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2003;45:13–17. [PubMed] [Google Scholar]
- 76.Suseelal T, John KR, Brown A, et al. . A study to assess the effectiveness of home based aerobic training, muscle strengthening and stretching exercise on self management among individuals with diabetes mellitus (DM) at selected villages in Kancheepuram district, Tamil Nadu. Int J Pharm Clin Res 2016;8:133–6. [Google Scholar]
- 77.Toufan M, Afrasiabi A. Benefits of cardiac rehabilitation on lipid profile in patients with coronary artery disease. Pakistan J. of Biological Sciences 2009;12:1307–13. 10.3923/pjbs.2009.1307.1313 [DOI] [PubMed] [Google Scholar]
- 78.van Rooijen AJ, Rheeder P, Eales CJ, et al. . Effect of exercise versus relaxation on haemoglobin A1c in black females with type 2 diabetes mellitus. QJM 2004;97:343–51. 10.1093/qjmed/hch061 [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Cai C, Padhye N, et al. . A behavioral lifestyle intervention enhanced with Multiple-Behavior self-monitoring using mobile and connected tools for underserved individuals with type 2 diabetes and comorbid overweight or obesity: pilot comparative effectiveness trial. JMIR Mhealth Uhealth 2018;6:e92 10.2196/mhealth.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wattana C, Srisuphan W, Pothiban L, et al. . Effects of a diabetes self-management program on glycemic control, coronary heart disease risk, and quality of life among Thai patients with type 2 diabetes. Nurs Health Sci 2007;9:135–41. 10.1111/j.1442-2018.2007.00315.x [DOI] [PubMed] [Google Scholar]
- 81.Wayne N, Perez DF, Kaplan DM, et al. . Health coaching reduces HbA1c in type 2 diabetic patients from a Lower-Socioeconomic status community: a randomized controlled trial. J Med Internet Res 2015;17:e224 10.2196/jmir.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiyono WH, Riyadi J, Yunus F, et al. . The benefit of pulmonary rehabilitation against quality of life alteration and functional capacity of chronic obstructive pulmonary disease (COPD) patient assessed using St George’s respiratory questionnaire (SGRQ) and 6 minutes walking distance test(6MWD). Med J Indones 2006;15:165–72. [Google Scholar]
- 83.Yadav A, Singh S, Singh KP, et al. . Effect of yoga regimen on lung functions including diffusion capacity in coronary artery disease patients: a randomized controlled study. Int J Yoga 2015;8:62–7. 10.4103/0973-6131.146067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yadav RK, Sharma R, Borah D, et al. . Efficacy of modified constraint induced movement therapy in the treatment of hemiparetic upper limb in stroke patients: a randomized controlled trial. J Clin Diagn Res 2016;10:YC01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yogendra J, Yogendra HJ, Ambardekar S, et al. . Beneficial effects of yoga lifestyle on reversibility of ischaemic heart disease: caring heart project of international board of yoga. J Assoc Physicians India 2004;52:283–9. [PubMed] [Google Scholar]
- 86.Zhang L, Zhang L, Wang J, et al. . Community health service Center-Based cardiac rehabilitation in patients with coronary heart disease: a prospective study. BMC Health Serv Res 2017;17:128 10.1186/s12913-017-2036-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shields GE, Wells A, Doherty P, et al. . Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart Br Card Soc (Epub ahead of print 13 April 2018). [DOI] [PMC free article] [PubMed]
- 88.McIntyre D, Thiede M, Dahlgren G, et al. . What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Soc Sci Med 2006;62:858–65. 10.1016/j.socscimed.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 89.Jamison DT, Feachem RG, Makgoba MW, et al. . Disease and Mortality in Sub-Saharan Africa. 2nd ed. Washington (DC): World Bank (eds), 2006. Available: http://www.ncbi.nlm.nih.gov/books/NBK2279/ [Accessed 10 Dec 2018]. [PubMed]
- 90.Early F, Wellwood I, Kuhn I, et al. . Interventions to increase referral and uptake to pulmonary rehabilitation in people with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:3571–86. 10.2147/COPD.S172239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sacks E, Morrow M, Story WT, et al. . Beyond the building blocks: integrating community roles into health systems frameworks to achieve health for all. BMJ Glob Health 2019;3:e001384 10.1136/bmjgh-2018-001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levack WM, Malmivaara A, Meyer T, et al. . Methodological problems in rehabilitation research. Report from a Cochrane rehabilitation methodology meeting. Eur J Phys Rehabil Med 2019;55:319–21. 10.23736/S1973-9087.19.05811-8 [DOI] [PubMed] [Google Scholar]
- 93.Guthold R, Stevens GA, Riley LM, et al. . Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. The Lancet Global Health 2018;6:e1077–86. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001833supp001.pdf (180.8KB, pdf)
bmjgh-2019-001833supp002.pdf (457.8KB, pdf)
bmjgh-2019-001833supp004.pdf (200.4KB, pdf)
bmjgh-2019-001833supp003.pdf (280.8KB, pdf)