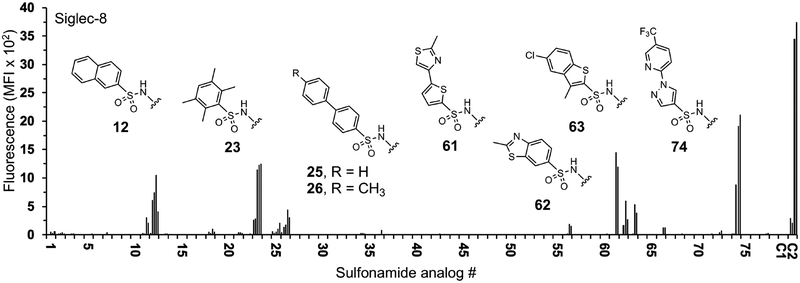
Figure 1.
Binding of recombinant Siglec-8 to the α2,3-sialyl sulfonamide analog array. Arrays were screened to identify C-9 substituents of sialic acid that show increased binding compared to control glycan C1. Human Siglec-8 COMP (10 μg/ml) was pre-complexed with anti-penta-histidine IgG-Alexa Fluor 488 (5 μg/ml). The complexed proteins were overlaid onto the array. After incubation the slides were washed then scanned for fluorescence. Analogs 1-78 correspond to the groups listed in Table S1. Shown is mean fluorescence intensity of Siglec-8 binding. Each glycan was printed at 100, 20, 4, 0.8, and 0.16 μM in 4 replicates each (increasing concentration from left to right). The controls are Neu5Acα2–3Galβ1–4GlcNAc (C1) and 6′-O-sulfo Neu5Acα2–3Galβ1–4GlcNAc (C2).