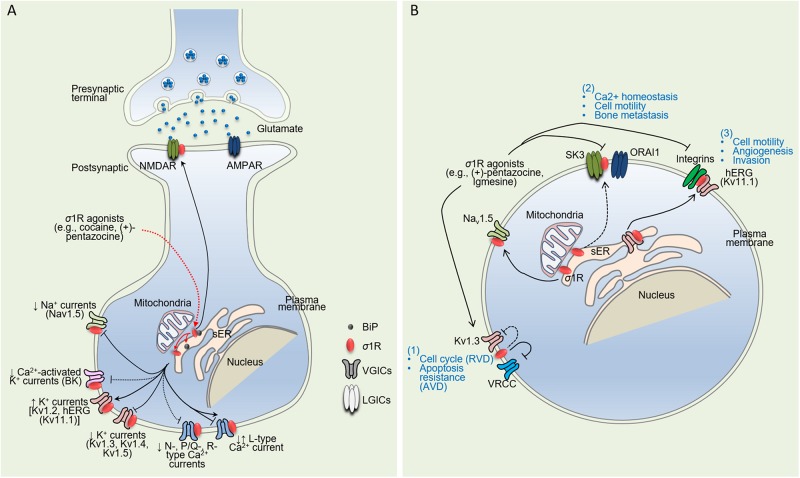
FIGURE 1.
Schematic diagram illustrating direct σ1R-dependent regulation of ion channels in neuronal and cancer cells. (A) Upon ligand stimulation [e.g., cocaine, (+)-pentazocine, PRE-084], σ1R dissociates from binding immunoglobulin protein (BiP), another endoplasmic reticulum (ER) chaperone protein, and then translocates from the mitochondrion-associated ER membrane (MAM, interface between mitochondrion and ER) to the ER and plasmalemma. Acting as an interorganelle signaling modulator, σ1R regulates a variety of functional proteins, both directly and indirectly. Here are represented only the regulations mediated by direct interaction with the targets. Pointed and flathead arrows indicate positive and negative regulations respectively. Unbroken and dashed lines indicate direct and indirect evidence for σ1R:Ion channels physical interactions. On the one hand, σ1R upregulates ion channel expression at the plasma membrane either through the regulation of subunit trafficking activity (hERG) (Crottès et al., 2011) or a mechanism that remains unidentified (Kv1.2) (Kourrich et al., 2013; Delint-Ramirez et al., 2018). σ1R activation by (+)-SKF 10,047 enhances binding with NMDARs, a mechanism that may play a role in NMDAR subunits trafficking to the cell surface (Balasuriya et al., 2013; Pabba et al., 2014). On the other hand, σ1R inhibits ion currents through modulation of target’s biophysical properties (Kv1.3, Kv1.4) (Aydar et al., 2002; Kinoshita et al., 2012) and likely trafficking mechanisms (Nav1.5) (Johannessen et al., 2009; Balasuriya et al., 2012). This can occur through both ligand-independent (Kv1.3, Kv1.4, Kv1.5) (Aydar et al., 2002; Kinoshita et al., 2012) and ligand-dependent mechanisms (Kv1.4, Kv1.5) (Aydar et al., 2002). σ1R can both enhance (Sabeti et al., 2007) and inhibit (Tchedre et al., 2008) L-type Ca2+ currents. Adapted from Kourrich (2017). (B) By shaping cancer cell electrical signature, σ1R participates to cancer hallmarks. (1) σ1R functionally modulates VRCC and K+ channels restricting cell sensitivity to AVD without altering cell cycle progression; (2) σ1R binds SK3 channel and promotes the formation of SK3:ORAI1 complexes within cholesterol-rich nanodomains responsible for increased Ca2+ influx and migration potency; and (3) σ1R dynamically associates hERG channels to integrins upon cell stimulation by ECM triggering motility, angiogenesis and invasiveness.