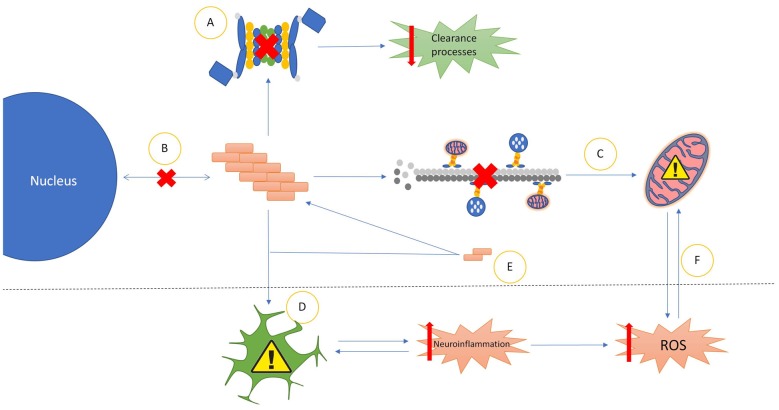
Figure 1.
Common properties of prionoid disease pathways. (A) Most prionoid proteins have been associated with inhibition of the cell’s autophagic machinery, particularly the lysosomal autophagy and ubiquitin-proteasome systems. This results in inhibited cellular clearance processes, enabling greater accumulation of aggregates. (B) Aggregated proteins often mislocalize into aberrant cellular compartments. In amyotrophic lateral sclerosis (ALS), TAR DNA-binding protein 43 (TDP-43) and fused in sarcoma (FUS) mislocalize from the nucleus to the cytoplasm. Amyloid-β in Alzheimer’s diseases (AD), α-synuclein in Parkinson’s disease (PD) and mutant superoxide dismutase-1 (mSOD1) in ALS mislocalize into the mitochondria. In Huntington’s disease (HD), Huntingtin (Htt) mislocalizes into the nucleus. (C) Many Neurodegenerative Disease (ND) processes involve the disruption of microtubule-mediated transport of various cellular components, particularly mitochondria. This often results in incorrect distribution of mitochondria, enhancing mitochondrial dysfunction. (D) Glial cells exert various neurotoxic and neuroprotective effects. In NDs these are often either insufficient to control pathological processes or subverted to enhance pathological spread or severity. The most common mechanism of this is increased neuroinflammatory activity, leading to the production of high levels of neurotoxic reactive oxygen species (ROS). The resultant oxidative injury enhances glial activation, compounding the effects of the pathology. (E) Soluble oligomeric species of prionoid proteins are often present in the cytoplasm of infected cells. While typically incapable of seeding aggregates, they are recruited to aggregates in order to accelerate their growth. There is evidence that oligomeric prionoids exert neurotoxic effects. Oligomers may contribute to the spread of pathology through uptake by microglia after being exocytosed. (F) Dysfunction in mitochondria leads to increased production of neurotoxic ROS. The downstream effects of ROS activity can damage mitochondria, resulting in a self-reinforcing cycle.