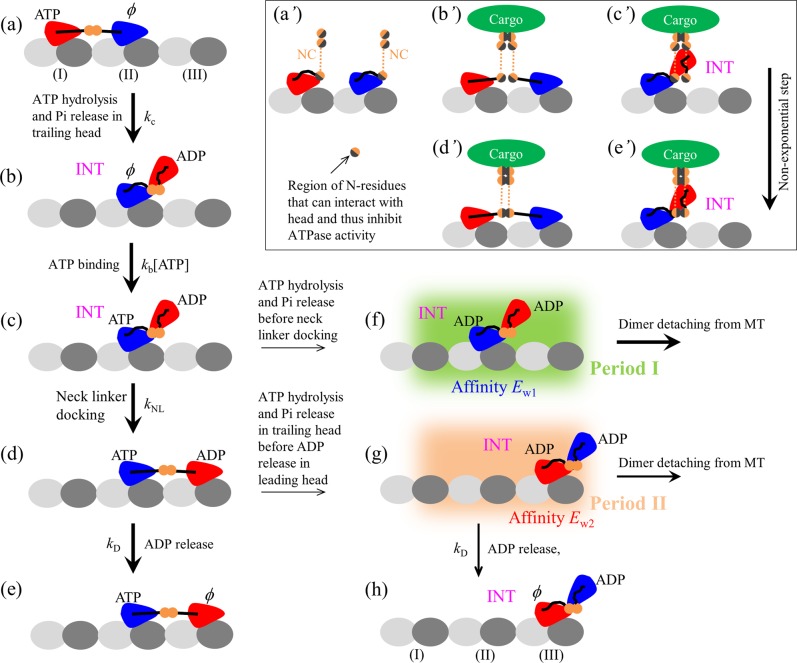
Figure 1.
Schematic of a typical forward stepping of dimeric kinesin-1 and kinesin-3. (a) The trailing head in ATP state binds strongly to binding site I on MT while the leading head in nucleotide-free (ϕ) state binds strongly to site II. The rate of ATP hydrolysis and Pi release of the trailing head with the forward orientation of the NL is much higher than that of the leading head without the forward orientation of the NL. (b) After ATP hydrolysis and Pi release in the trailing head, due to the very weak affinity (Ew1) between the ADP-head and the local site I, the ADP-head detaches easily from site I and diffuses rapidly to the INT position relative to the MT-bound head, where the two heads have a high affinity. (c) ATP binds to the MT-bound ϕ-head. (d) The NL docking of MT-bound head takes place, weakening the interaction between the two heads, and the tethered ADP-head then diffuses rapidly to the forward site III on MT. Note that the NL docking provides an energy barrier ENL to prevent the tethered ADP-head from moving backward. (e) Stimulated by MT, ADP is released from the leading head. State in (e) is the same as that in (a) except a forward step was made. (f) From (c), ATP hydrolysis and Pi release can also occur occasionally in the MT-bound head before its NL docking. Before the affinity of the MT-bound ADP-head for the local site II changes from Ew1 to Ew2 (called Period I), the dimer can detach easily from MT by overcoming the very weak affinity Ew1. (g) From (d), ATP hydrolysis and Pi release can also occur occasionally in the trailing head before ADP is released from the leading head. The dimer also has a large probability to detach from MT before ADP is released from the MT-bound head (called Period II) by overcoming weak affinity Ew2. (h) From (g), the dimer has not detached from MT until ADP is released from the MT-bound head. State in (h) is the same as that in (b) except a forward step was made. Inset (a’ – e’) is the schematic illustration of the intermolecular interaction between two NC segments and intramolecular interaction of N-residues with the head during cargo-mediated dimerization of kinesin-3. (a’) Before cargo-mediated dimerization, the intramolecular interaction of the N-residues with head inhibits its ATPase activity. (b’,c’) After cargo binding, firstly the C-terminal residue (adjacent to the cargo) in one NC helix forms an intermolecular interaction with the corresponding residue in another NC helix. Then, the intermolecular interactions between residues in the two helixes form successively. Before formation of the intermolecular interaction between N-residues in the two helixes, in the state with two heads bound to MT, the stretching of the two NLs causes the N-residues to be away from their heads, with no intramolecular interaction between the N-residues and head. The two heads thus have the ATPase activity (b’). In INT state, the N-residues are close to the head and the intramolecular interaction between them is present, inhibiting the ATPase activity of the MT-bound head (c’). (d’, e’) After complete formation of the coiled-coil between two NC helixes, the formation of the intermolecular interaction between N-residues in the two helixes prevents the intramolecular interaction between the N-residues and MT-bound head in both the state with the two heads bound to MT (d’) and INT state (e’). Thus, in both states the MT-bound head has the ATPase activity.