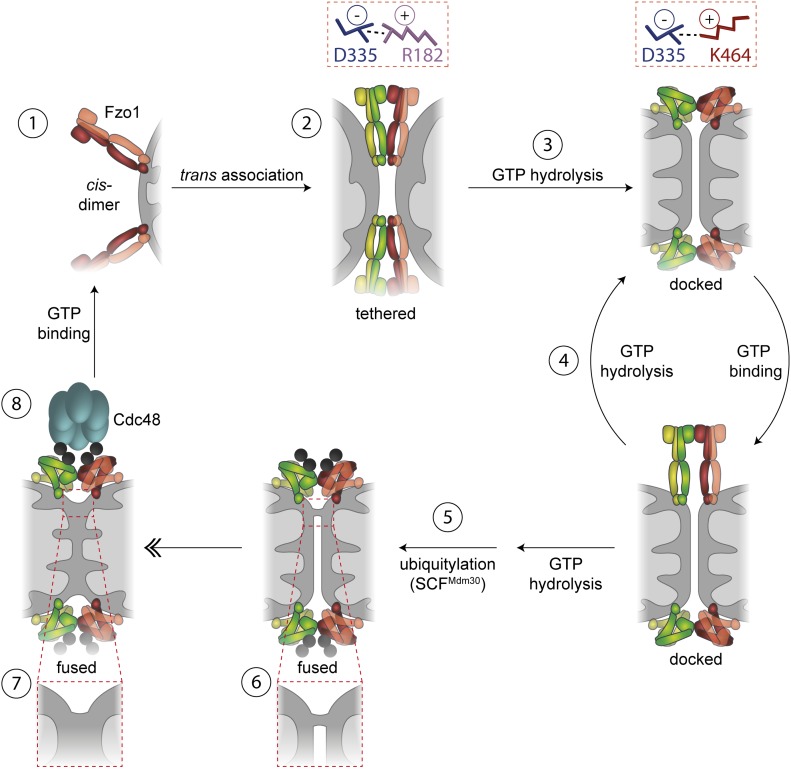
Figure 5. Integrated model for mitochondrial OM fusion.
Model for OM fusion. GTP-bound Fzo1 dimers localize at the OMM (1). Fzo1 trans association leads to formation of the tethering complex, which depends on dynamic salt bridge interactions (2). GTP hydrolysis shifts the salt bridge from R182 to K464 and thereby drives conformational changes on Fzo1 (3) eventually promoting membrane curvature and formation of the docked stage. Recurring cycles of GTP binding and hydrolysis (4) allow membrane approximation and ubiquitylation of Fzo1 by SCFMdm30 (5), eventually allowing local lipid merging (6), which rapidly expands for complete fusion of the two OMs (7). After membrane merging, Fzo1 ubiquitylation is controlled by Cdc48, possibly leading to complex disassembly (8).