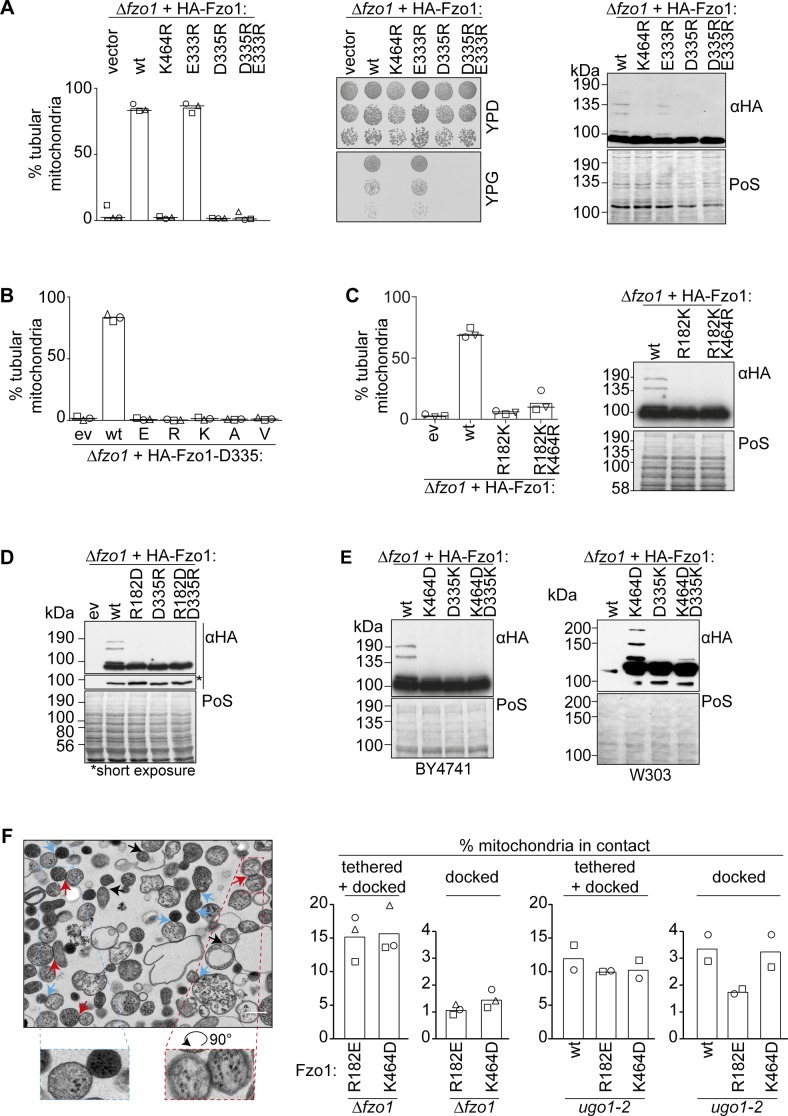
Figure S2. Identification of critical residues in Fzo1.
(A) D335 is required for Fzo1 functionality. Mitochondrial morphology (left), respiratory capacity (middle), and ubiquitylation (right) of ∆fzo1 cells expressing the indicated HA-Fzo1 variants, as indicated in Fig 1. (B) Stringent requirement of an asparagine at position 335. Quantification of mitochondrial morphology as in Fig 1D of ∆fzo1 cells expressing the wt or mutant variants of HA-Fzo1D335, as indicated. (C) R182 and K464 cannot be exchanged. Quantification of mitochondrial morphology as in Fig 1D (left) and ubiquitylation as in Fig 1B (right) of ∆fzo1 cells expressing HA-Fzo1 wt or mutant variants. (D, E) Salt bridge charge swap with D338 and either R182 in (D) or K464 in (E). Indicated mutations of Fzo1 were expressed in ∆fzo1 cells and Fzo1 ubiquitylation analyzed as in Fig 1B. In (E), a BY4741 (left) or W303 (right) background were used. PoS, PonceauS staining. (F) In vitro analysis of mitochondrial contact sites. Mitochondria were purified from ∆fzo1 cells expressing HA-Fzo1R182E or HA-Fzo1K464D or from ∆fzo1 ugo1-2 cells expressing HA-Fzo1, HA-Fzo1R182E, or HA-Fzo1K464D and analyzed by TEM. Engaged contact sites, meaning tethering (blue arrows) plus docking events (red arrows) we quantified. Loose contact sites were not regarded for quantification (black arrows) Scale bar: 300 nm (left). At least 1,000 mitochondria were quantified (right), including mean (bars) and individual experiments (circles, squares, and triangles).