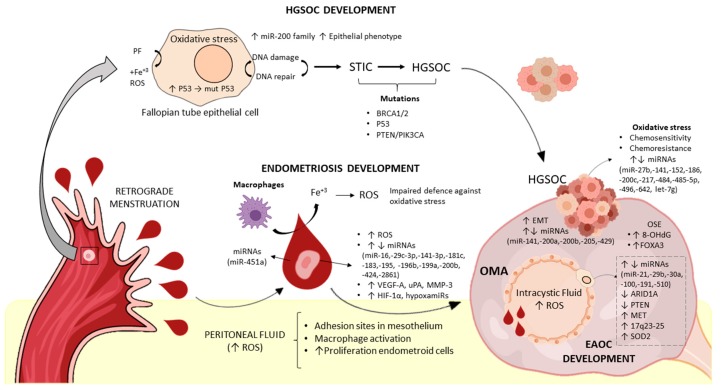
Figure 3.
Global vision of aetiopathogenic mechanisms leading to endometriosis, EAOC and HGSOC development. Regarding HGSOC development, oxidative stress contributes to fallopian tube epithelial cells alterations, through the action of ROS. Repeated cycles of DNA damage and repair produce mutations in driver genes BRCA1/2, P53, PTEN and PIK3CA. Additionally, miRNA deregulation contributes to tumour progression. Once the malignant lesion is established in the ovary, oxidative stress is initially involved in first-line chemotherapy mechanism of action, although excessive oxidative stress is linked to tumour chemoresistance. Regarding endometriosis development, refluxed endometrial cells from patients show some features predisposing them to the development of this condition (i.e., increased angiogenesis and proteolysis, disbalanced miRNAs profile, etc). Upon menstruation, endometrial cells lose their blood supply and activate hypoxia-responsive miRNAs (hypoxamiRs) that together with erythrocyte-derived miRNAs contribute to the development of the condition. Blood decomposition by pelvic macrophages contribute to ROS production, which alters the peritoneal microenvironment to enhance endometrial cells attachment and proliferation. Finally, the intra-cystic fluid of OMAs presents with higher levels of ROS, triggering subsequent events such as miRNAs disbalance, decreased expression of ARID1A and PTEN, amplification of MET and 17q24–25, and increased generation of SOD2, all of which enhance the development of EAOC. Abbreviations: EAOC, endometriosis-associated ovarian cancer; HGSOC, High-Grade Serous Ovarian Cancer; STIC, Serous Tubal Intraepithelial Carcinoma; OSE, Ovarian Surface Epithelium; OMA, Ovarian endometrioma; ROS, Reactive Oxygen Species; PF, Peritoneal Fluid; BRCA, Breast Cancer gene; PTEN, Phosphatase and tensin homolog; PIK3CA, Phosphatidylinostil-4,5 Biphosphonate 3-Kinase Catalytic Subunit Alpha; VEGF-A, Vascular Endothelial Growth Factor A; uPA, Urokinase-type plasminogen Activator; MMP-3; Matrix Metallopeptidase 3; HIF-1α; Hypoxia-inducible factor 1-alpha; ARID1A, AT-Rich Interaction Domain A; MET, mesenchymal-to-epithelial transcription factor; EMT, Epithelial-Mesenchymal Transition; SOD2, Superoxide Dismutase 2; FOXA3, Hepatocyte Nuclear Factor 3-gamma; 8-OHdG, 8-Oxo-2′-deoxyguanosine.