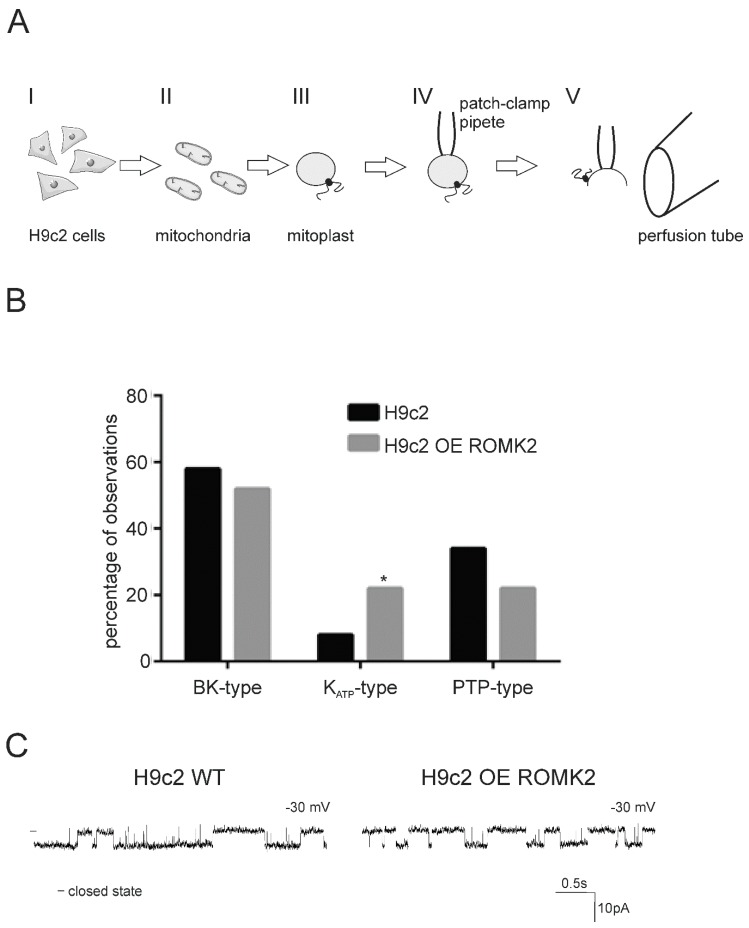
Figure 1.
Procedure of the mitochondrial patch-clamp experiment and summary of recorded channel activities. (A) Mitoplasts (mitochondria without the outer membrane) were prepared from mitochondria of the H9c2 WT and renal outer medullary potassium channel (ROMK2) overexpressing H9c2 cells (I). Mitochondria were added to the hypotonic solution to induce swelling and outer membrane breakage. Isotonicity was restored by adding a hypertonic solution (II and III). A free-floating mitoplast was attached to a glass pipette (IV). Ion current was measured in an inside–out configuration. The modulators of the channels were added through a perfusion system (V). See more information in the Experimental Procedures section. (B) A comparison of mitochondrial ion channel activities from H9c2 WT (black bars) and H9c2 OE ROMK2 (grey bars) cell lines. The analysis was based on n = 50 recorded channels (all types) for each cell line. Data were obtained from around 30 independent mitochondrial isolations for each cell line. The number of observed KATP-type channels in mitochondria H9c2 OE ROMK2 was significantly higher. The chi-squared statistic was 3.8431, * p < 0.05. (C) A comparison of ion channel kinetics classified in Panel B as mitoKATP in the H9c2 WT cells and H9c2 cells overexpressing ROMK2. Recordings were performed at −30 mV.