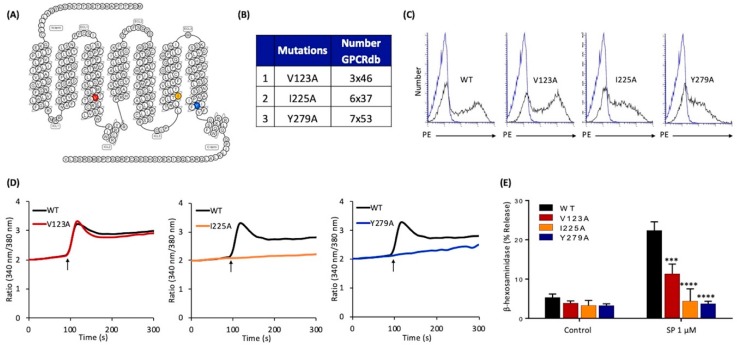
Figure 2.
Effects of mutations at MRGPRX2’s highly conserved positions within transmembrane domains (V123A, I225A, and Y279A) on cell surface expression, SP-induced Ca2+ mobilization, and degranulation in transiently transfected RBL-2H3 cells. (A) Snake diagram of secondary structure of MRGPRX2. Each circle represents amino acid residue with one letter code. Solid red, yellow, and blue backgrounds denote the residues at positions 3x46 (V123), 6x37 (I225), and 7x53 (Y279), respectively; (B) amino acid change for each MRGPRX2 mutant.; (C) RBL-2H3 cells transiently expressing wild-type (WT)-MRGPRX2 and its mutants were incubated with phycoerythrin (PE)-anti-MRGPRX2 antibody and cell surface receptor expression was determined by flow cytometry. Representative histograms for WT/mutant (black line) and control untransfected cells (blue line) are shown; (D) cells expressing WT-MRGPRX2 and its mutants were loaded with Fura-2 and intracellular Ca2+ mobilization in response to SP (1 μM) was determined. Data shown are representative of three independent experiments; (E) cells were exposed to a buffer (control) or SP (1 μM) for 30 min, and β-hexosaminidase release was determined. All data points are the mean ± SEM of at least three experiments performed in triplicate. Statistical significance was determined by a nonparametric t-test. *** p ≤ 0.001 and **** p ≤ 0.0001.