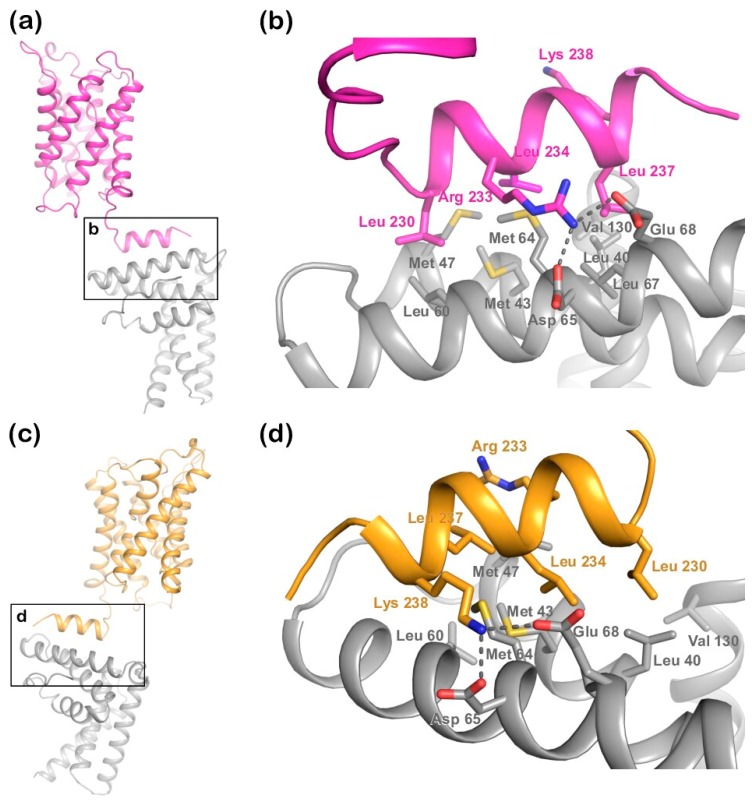
Figure 4.
Structural representation of the two best docking models from HADDOCK. (a) Cluster 3 solution with the C-terminal helix of AQP2 (magenta) binding to the first MIT-domain of the LIP5 N-terminal domain (grey); (b) zoomed-in view of the interacting surface in (a) showing that in this solution, LIP5 binds AQP2 in a similar manner as in the LIP5-CHMP1B complex (Figure 2b,c); (c) cluster 2 solution showing how the AQP2 (orange) C-terminal helix binds the LIP5 N-terminal domain in the opposite orientation; (d) Zoom-in view of the interacting surface in (c). In (b,d), all residues corresponding to the binding interface in the LIP5-CHMP1B complex are shown in stick representation. Predicted hydrogen bonds are depicted as dotted lines.