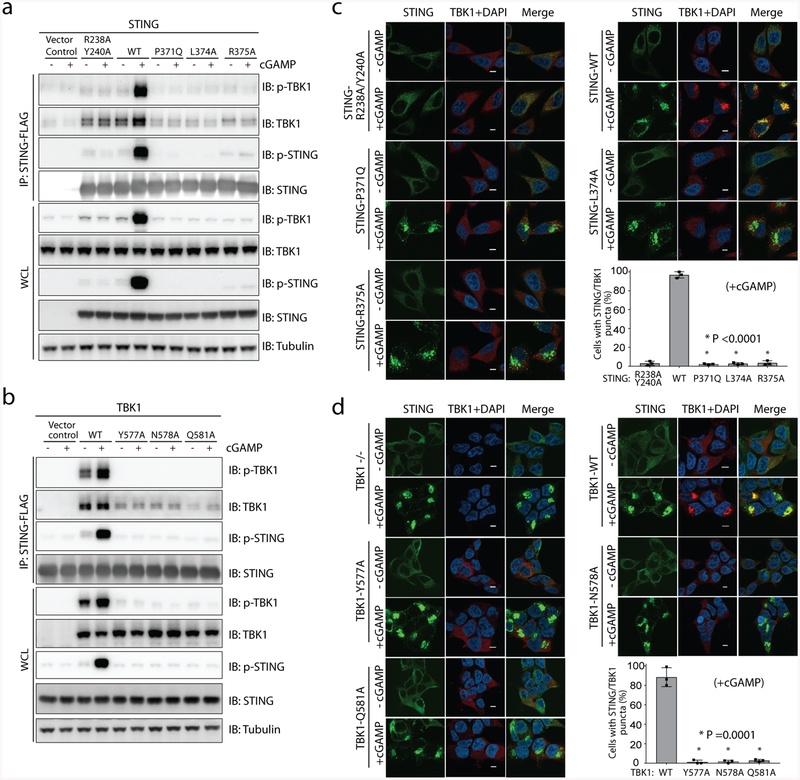
Fig. 3 |. The interface between TBK1 and the STING C-terminal tail is required for TBK1 binding and colocalization with STING in cells.
a, b, Mutations of interface residues in either STING (a) or TBK1 (b) abolish cGAMP-induced interaction between STING and TBK1, as well as the phosphorylation of both proteins, as shown by immunoblotting. Cells used in a, b are the same as those used in Fig. 2h, i, respectively. After cGAMP stimulation, immunoprecipitation was carried out to examine the interaction between STING and TBK1. Data are representative of three independent biological replicates. WCL, whole-cell lysate. c, Mutants of interface residues in STING diminish colocalization of TBK1 with cGAMP-induced puncta of STING in cells. HeLa cells deficient in cyclic GMP–AMP synthase and expressing the wild type or mutant constructs of STING were stimulated with cGAMP (1 μM) for 1 h, and then subjected to immunostaining for STING (green, 488 nm), TBK1 (red, 568 nm) and DAPI. Representative confocal images of cells with or without cGAMP stimulation are shown. Scale bars, 5 μm. d, Mutants of interface residues in TBK1 diminish colocalization of TBK1 with cGAMP-induced puncta of STING in cells. HEK293T cell lines that stably express wild-type or mutant TBK1 (as in b) were stimulated with cGAMP (1 μM) for 1 h and subjected to immunostaining (as in c). Scale bars, 5 μm. In both c, d, the percentage of cells with STING and TBK1 colocalization was quantified from at least 150 cells for each group. Data are mean ± s.d. and representative of three independent biological replicates.