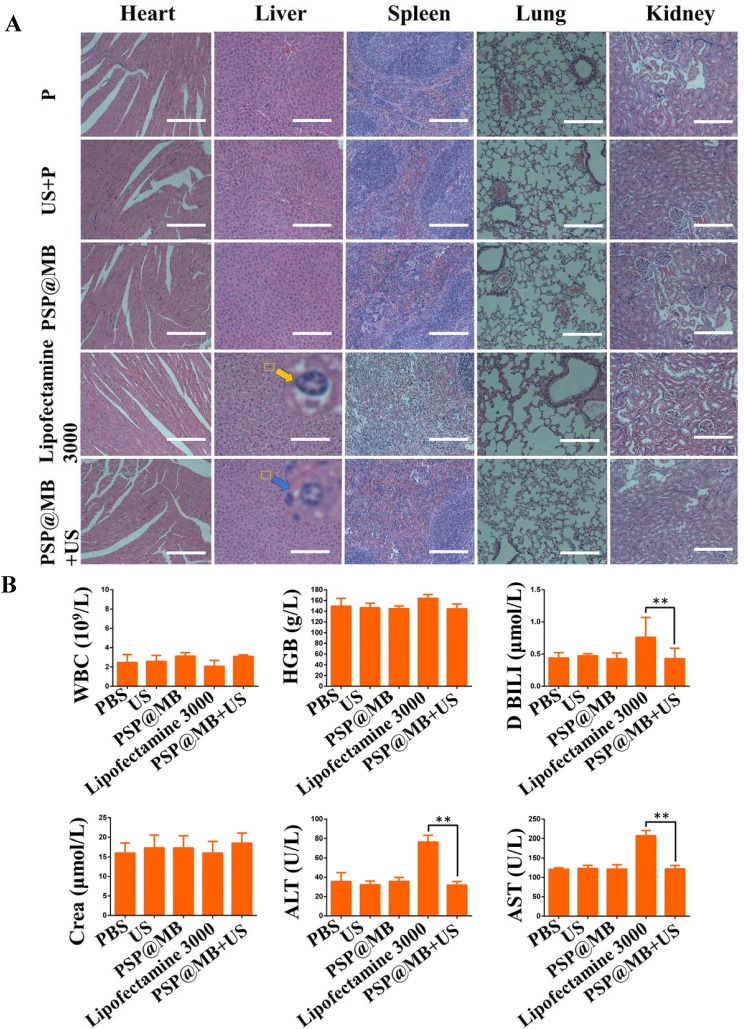
Figure 7.
Biosafety evaluation.
Notes: (A) Histological examination of hematoxylin and eosin (H&E) staining of vital organ (heart, liver, spleen, lung, kidney) sections. The result of H&E staining assay showed symptoms of swell, fatty and vacuolar in the liver cells treated by lipofectamine 3000 (yellow arrow). The PSP@MB+US group (blue arrow) demonstrated the normal morphology of liver cells compared with plasmid group (scale bar=100 μm). (B) The liver functions (ALT and AST), the renal functions (Crea), Direct Bilirubin (D BILI) and routine blood parameters (hemoglobin and white blood cell) were tested. All the biochemical parameters in serum and routine blood test remained at a normal range in PSP@MB and ultrasound group. Compared with PSP@MB and ultrasound group, the activity of ALT, AST and D BILI were increased significantly in lipofectamine 3000 group. The histopathological and liver functions results exhibited that PSP@MB displayed better biocompatibility than lipofectamine 3000 at a certain concentration in vivo. Data are represented as mean ± standard deviation; n = 3; **P<0.01.