Figure 2.
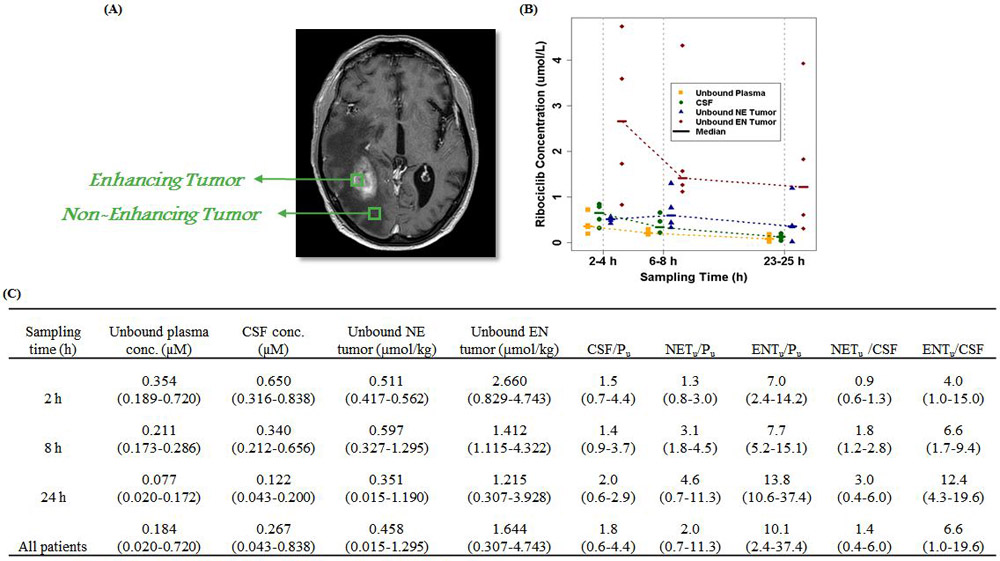
CNS pharmacokinetics of ribociclib in glioblastoma patients (A) Representative imaging indicating enhancing and non-enhancing region of the tumor. (B) Observed unbound ribociclib concentrations in plasma, CSF, non-enhancing, and enhancing glioblastoma tumor regions at 2 to 24 hours after oral administration of the fifth dose of ribociclib. Patients were treated with a daily oral dose of 900 mg for 5 days, and all samples were collected on day 5. Symbols represent observed concentrations, and dash lines represent the median concentration-time profiles. (C) Summary of pharmacokinetics characteristics of CNS ribociclib in glioblastoma patient. Data are presented as the median (range). Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; NE, non-enhancing; EN, enhancing; CSF/Pu, CSF-to-plasma unbound drug concentration ratio; NETu/Pu, non-enhancing tumor-to-plasma unbound drug concentration ratio; ENTu/Pu, enhancing tumor-to-plasma unbound drug concentration ratio; NETu/CSF, non-enhancing tumor-to-CSF unbound drug concentration ratio; ENTu /CSF, enhancing tumor-to-CSF unbound drug concentration ratio.
