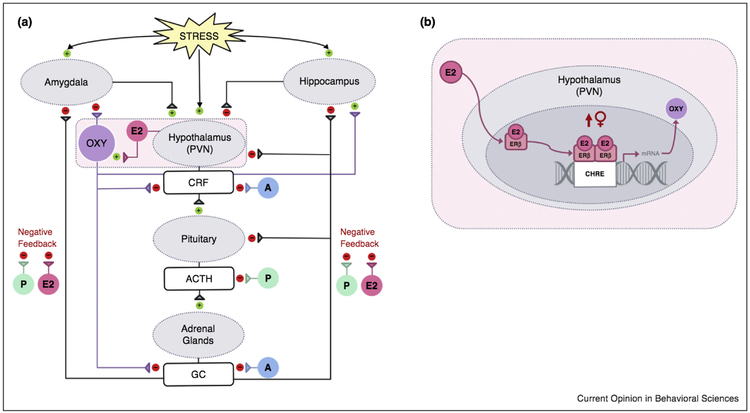
Figure 1.
(a) Model of hypothesized interactions between oxytocin, gonadal steroids, and stress systems. In response to acute stress, a neuroendocrine cascade is triggered which promotes CRF secretion within the PVN of the hypothalamus that leads to the release of ACTH from the anterior pituitary which ultimately promotes the production and release of glucocorticoids (GC) from the cortices of the adrenal glands. Centrally, exposure to stress recruits areas within stress-sensitive neural pathways including the amygdala and hippocampus, both of which regulate neuroendocrine responses to stress. Oxytocin administration prior to stress inhibits this neuroendocrine response, reducing the secretion of CRF and glucocorticoids. In the CNS, oxytocin administration attenuates stress-related activity within the amygdala and appears to protect against stress-induced alterations in hippocampal synaptic plasticity. Circulating gonadal hormones including androgens (A), estrogens (E2), and progesterone (P) have the potential to affect HPA axis activity and modulate oxytocin neuron functioning which may contribute to sex differences in stress responsiveness. In general, androgens including testosterone reduce stress-induced glucocorticoid release, in part via androgen inhibition of CRF expression. Ovarian steroid effects on HPA axis activity are more complex. In rodents, estradiol and progesterone inhibit glucocorticoid negative feedback that may impair downregulation of the stress response. Conversely, estradiol can decrease neuroendocrine responses to stress via its actions on estrogen receptor beta (ERβ). (b) Estrogen receptor beta regulation of oxytocin mRNA expression. Within the PVN, estradiol binds to nuclear ERβ which rapidly dimerizes and binds to the composite hormone response element (CHRE) of the oxytocin gene. This enhances oxytocin transcript expression and increases oxytocin peptide levels which can ultimately lead to overall reductions in stress-related HPA axis activity. Although the mechanisms are not yet understood, it appears that females may be more sensitive to these effects.
Adapted from Acevedo-Rodriguez et al. [24].