Abstract
Background
Children infected with human immunodeficiency virus (HIV) are at high risk of acquiring intestinal parasitic infections. This study aimed to determine the magnitude of Cryptosporidium and other intestinal parasitic infections and concomitant threats among HIV-infected children.
Methods
A hospital-based cross-sectional study was carried out at three antiretroviral therapy clinics in southern Ethiopia from February 2016 to June 2017 in 384 HIV positive children. Socio-demographic and clinical data were collected using structured questionnaires. Direct stool microscopic examination and modified Zeihl–Neelsen staining technique to identify parasites. Chi-square test was conducted to determine the real predictors of the infection. Significant association was considered when p-value <0.05 at 95% CI.
Results
The overall magnitude of intestinal parasitic infections among the study population was 16.9% (95% CI: 13.0–20.8%). The most predominant parasitic infections were Cryptosporidium spp. (9.6%) and the least was Taenia spp. (0.78%). Diarrheal status (χ2=7.653, df=2, p=0.022) was detected to be the only significant associated variable.
Conclusion
Cryptosporidium infection was found to be the most common intestinal parasitosis among HIV-infected children. Routine screening service for Cryptosporidium and other intestinal parasites is important in the clinical management of HIV-infected children.
Keywords: intestinal parasites, Cryptosporidium, HIV/AIDS, Southern Ethiopia
Background
Cryptosporidiosis is a diarrheal disease caused by the water-borne parasite, Cryptosporidium. The predominant symptom of cryptosporidiosis is watery diarrhea. In people with immunocompetent, the infection typically lasts approximately 2 weeks. However, those with decreased or compromised immune systems are at greater risk for developing serious, chronic diarrhea.1
Human immunodeficiency virus (HIV) was the most common community-acquired infection among approximately 2.1 million children under 15 years old globally in 2016.2 In people with HIV-infection, Cryptosporidium is an opportunistic infection and is considered an AIDS-defining illness.3 In addition to Cryptosporidium, other intestinal parasites are associated with both acute and chronic diarrhoea which reduces the quality of life among HIV-infected patients.4 In developing countries, cryptosporidiosis is prevalent and has been demonstrated as high as 45% among children with associated impaired growth, malnutrition, and immune suppression.5 The lack of clean and potable water, poor hygiene, and unsanitary environmental conditions are generally responsible for the prevalence of intestinal parasites in most African nations.4 Parasites such as Cryptosporidium spp., Isospora belli, Giardia duodenalis, Entamoeba spp., Cyclospora cayetanensis, Blastocystis spp., Strongyloides stercolaris, and Ascaris lumbricoides are the most commonly reported among HIV-infected patients.6,7
Children in emerging nations such as Africa, Asia, and Latin America are especially susceptible to chronic parasitic infections because they are immunologically naïve and often malnourished.8,9 Cryptosporidiosis has been shown to play a fundamental role in infantile malnourishment in unindustrialized nations,10,11 and has been associated with diminished growth in late childhood.12 Combination antiretroviral therapy (cART) has been found to improve physical, cognitive and nutritional outcomes among HIV-infected children and adolescents.13,14 Cryptosporidiosis among HIV-infected children while on cART may compromise the effectiveness of the regimens.15,16
In a study in Gambian children, those consumed stored water, presence of large family size and had contact with animal are at risk of Cryptosporidium infection.17 Another study from Cameron in under five children the presence of diarrhea was reported as concomitant treat for Cryptosporidium infection.18 In addition to this factors undernutrition, having previous hospitalization, older age and living in a day care institution was statically significant associated risk factors reported from Colombian children with HIV-infection.19 Therefore, this study evaluates the degree of opportunistic Cryptosporidium and other intestinal parasitic infections (IPIs) among children receiving cART and concomitant threat.
Materials And Methods
Study Design, Area And Period
A cross-sectional study of intestinal parasitic infection in HIV-infected children conducted in three public hospitals (Hawassa University Comprehensive Specialized Hospital (HUCSH), Adare General Hospital (AGH) and Wolyita Sodo Referral Hospital (WSRH)) in southern Ethiopia from February 2016 to June 2017. The hospitals are located in one of the administrative regions, the Southern Nations, Nationalities and Peoples’ Regional State of Ethiopia.
Study Populations
The study population was HIV-infected children who had regular follow-up visits at the HIV/ART clinic of each hospitals included for this study in one of their visit. Children who had received anti-parasitic treatment within a month prior to the time of data collection were excluded from the study. A total of 384 children (211 male and 173 female) met the inclusion criteria and were enrolled in the study. There were 217 children from HUCSH, 108 children from WSRH, and 59 children from AGH.
Data Collection
Socio-demographics, existing symptoms, and current or previous history of diarrhoea were collected using structured questionnaires. Diarrhoea was defined as a minimum of three watery or loose stools within 24 hrs. Diarrhoea lasting for 14 days was defined as chronic diarrhoea, and diarrhoea that lasted for fewer than 14 days was categorized as acute diarrhoea (Annex).20
Laboratory Diagnosis
A single stool specimen approximately 2mg was collected from each participant using screw caped plastic container on the same day of the interview. Unconcentrated stool samples were examined using direct microscopy (normal saline and iodine preparation) techniques to identify the presence of trophozoite/cyst, larvae, or ova of intestinal parasites. The modified Zeihl–Neelsen staining technique was performed to visualize Oocysts of intestinal coccidian parasites as described elsewhere.21 Four milliliter (4mL) blood sample was collected using ethylene diamine tetra acetic acid (EDTA) to determine the CD4 T cell count using fluorescence-activated cell sorting (FACS) machine based on flow cytometry principle.22 All the samples were processed at the study sites with the close supervision of principal investigator.
Data Analysis
Data entry and analysis was conducted with SPSS Version-20. Mean, range, and proportions were calculated to summarize data as appropriate. Variances among magnitudes were assessed using Pearson’s Chi-square test (χ2), and a P-value <0.05 was considered significant.
Ethical Consideration
The Institutional Review Board of the College of Medicine and Health Sciences, Hawassa University approved the study. Involvement was voluntary and informed written consent was obtained from caregivers of the study participants. Physicians managed those found to be infected with any pathogenic intestinal parasites.
Results
Sociodemographic Characteristics Of The Study Subjects
A total of 384 children (211 male and 173 female) were included for the study. The children were grouped by age: 1–6 years old (33), 7–12 years old (161) and 13–18 (190). Of those tested, 54.9% (211) were male and 87.2% (335) were urban residents. Most of their mothers had completed primary school 66.9% (257). Variables considered were the age and sex of the child as well as the educational background of the child’s mother as the most likely caregiver. The living environment (urban/rural) as well as source of drinking water and regular contact with animals was considered. However, there were no statical significantly associated variables for Cryptosporidium as well as to other intestinal parasites (Table 1).
Table 1.
Socio-Demographic Characteristics Of Study Subjects For The Study Of Cryptosporidium And Other Intestinal Parasites Among HIV Positive Children In Southern Ethiopia
| Variables | Frequency | Presence Of Any Parasite | χ2, df, (p-value) | Presence Of Cryptosporidium | χ2, df, (p-value) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | (%) | No. | % | |||
| Age groups years | 384 | 65 | 0.3882 (0.824) | 37 | 1.7592 (0.415) | |||
| 1–6 | 33 | 8.6 | 6 | 18.3 | 2 | 6.1 | ||
| 7–12 | 161 | 41.9 | 25 | 15.5 | 13 | 8.1 | ||
| 13–18 | 190 | 49.5 | 34 | 17.9 | 22 | 11.6 | ||
| Sex | 0.0381 (0.845) | 0.6561 (0.418) | ||||||
| Male | 211 | 54.9 | 35 | 16.6 | 18 | 8.5 | ||
| Female | 173 | 45.1 | 30 | 17.3 | 19 | 11.0 | ||
| Mother’s occupation | 10.9552 (0.141) | 0.3732 (0.830) | ||||||
| Government employee | 76 | 19.8 | 12 | 15.8 | 7 | 9.2 | ||
| NGO | 53 | 13.8 | 11 | 20.8 | 4 | 7.5 | ||
| Others | 213 | 66.4 | 42 | 16.5 | 26 | 10.2 | ||
| Mother’s education | 1.0572 (0.590) | 1.0422 (0.594) | ||||||
| Illiterate’s | 16 | 4.2 | 2 | 12.5 | 2 | 12.5 | ||
| Primary school (1–6) grade | 257 | 66.9 | 41 | 16 | 22 | 8.6 | ||
| 2nd school (7–12) grade | 111 | 28.9 | 22 | 19.8 | 13 | 11.7 | ||
| Residence | 0.2791 (0.598) | 0.0211 (0.885) | ||||||
| Urban | 335 | 87.2 | 58 | 17.3 | 5 | 10.2 | ||
| Rural | 49 | 12.8 | 7 | 14.3 | 32 | 9.6 | ||
Abbreviations: χ2, chi-square testing; df, degree of freedom; NGO, non-governmental organization.
Magnitude Of Intestinal Parasites
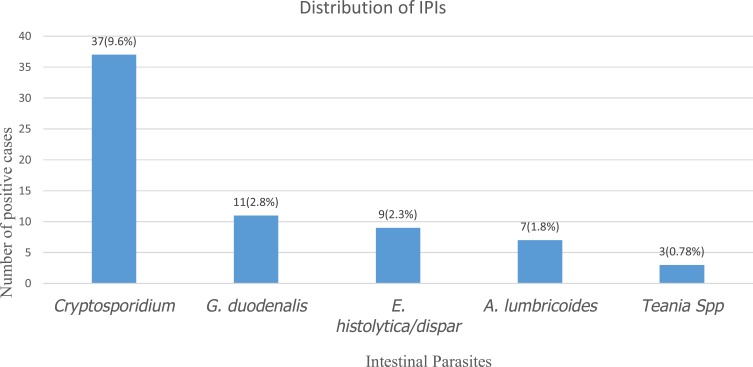
The overall intestinal parasitic infections rate was 16.9% (95% CI: 13.0–20.8%). Cryptosporidium was the most frequently identified 37 (9.6%) parasite, followed by G. duodenalis 11 (2.8%), E. histolytica/dispar 9 (2.3%), A. lumbricoides 7 (1.8%) and Taenia spp. 3 (0.78%) (Figure 1). Mixed parasites were observed in two cases that are Cryptosporidium occurred with G. duodenalis (1%), E. histolytica/dispar (1%) and A. lumbricoides (1%) as co-infection.
Figure 1.
Distribution of intestinal parasites among HIV positive children in Southern Ethiopia, February 2016 to June 2017.
Associated Risk Factors
The degree of diarrhea (absent/acute/chronic) and CD4 cells/µL levels were tracked from blood sample. There is a correlation between the source of drinking water and contact with animals with the degree of diarrhea, intestinal parasites and CD4 levels. Those children who reported drinking piped water (91.7%) and had no contact with animals (76%) were highly likely to report no diarrhea (81.8%). They had CD4 levels >500 cells//µL (64.8%). However, children who drink spring water (16.7%), and had animal contact (22.8%) had higher rates of intestinal parasites than lacks animal contact. These children were more likely to have acute diarrhea (31.7%) and CD4 levels <200 cells/µL. Diarrheal status was the only significantly associated variable with intestinal parasites (χ2=7.653, df=2, p=0.022) as presented in Table 2.
Table 2.
Assessment Of Risk Factors For Cryptosporidiosis And Other Intestinal Parasites Among HIV Positive Children In Southern Ethiopia
| Variables | Frequency | Presence Of Any Parasite | χ2, df, (p-value) | Presence Of Cryptosporidium | χ2, df, (p-value) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | (%) | No. | % | |||
| Drinking water source | 2.9862 (0.225) | 0.7122 (0.701) | ||||||
| Pipe | 352 | 91.7 | 33 | 9.4 | 33 | 9.4 | ||
| Hand pump | 20 | 5.2 | 2 | 10 | 2 | 16.7 | ||
| Spring | 12 | 3.1 | 2 | 16.7 | 2 | 10.0 | ||
| Animal contact | 2.9941 (0.084) | 0.2121 (0.645) | ||||||
| Yes | 92 | 24 | 21 | 22.8 | 10 | 10.9 | ||
| No | 292 | 76 | 44 | 15.1 | 27 | 9.2 | ||
| Diarrhea status | 7.6532 (0.022) | 0.5612 (0.755) | ||||||
| Absence | 314 | 81.8 | 49 | 15.6 | 30 | 9.6 | ||
| Acute | 41 | 10.7 | 13 | 31.7 | 5 | 12.2 | ||
| Chronic | 29 | 7.6 | 3 | 10.3 | 2 | 6.9 | ||
| CD4 level cells/µL | 1.5892 (0.452) | 2.0202 (0.364) | ||||||
| <200 | 31 | 8.1 | 7 | 22.6 | 5 | 16.1 | ||
| 200–499 | 104 | 27.1 | 20 | 19.2 | 11 | 10.6 | ||
| >500 | 249 | 64.8 | 38 | 15.3 | 21 | 8.4 | ||
There is also a correlation between low CD4 levels and parasitic infection rates. Children with CD4 count <200 cells/µL were more likely to have a parasite (22.6%) than those with CD4 levels of 200–499 cells/µL (19.2%) or CD4 >500 cells/µL (15.3%). In each group, the percentage of Cryptosporidium was consistent (16.1%, 10.6% and 8.4%, respectively). However, CD4 level is not statistically significant (Table 3).
Table 3.
Relation Of CD4 Count With Diarrheal Status For Cryptosporidiosis And Other Intestinal Parasites
| CD4 Count Cells/µL | Patient Data | Any Parasite | χ2, df, (p-value) | Cryptosporidium | χ2, (p-value) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Diarrheal Status | No. | % | No. | % | No. | % | |||
| <200 | None | 21 | 67.7 | 6 | 28.6 | 4 | 19 | ||
| Acute | 9 | 29 | 1 | 11.1 | 1 | 11.1 | |||
| Chronic | 1 | 3.2 | 0 | 0 | 0 | 0 | |||
| Total | 31 | 8.1 | 7 | 22.6 | 1.3361 (0.248) | 5 | 16.1 | 0.4101 (0.522) | |
| 200–499 | None | 85 | 81.7 | 13 | 15.3 | 8 | 9.4 | ||
| Acute | 12 | 11.5 | 6 | 50 | 2 | 16.7 | |||
| Chronic | 7 | 6.5 | 1 | 14.3 | 1 | 14.3 | |||
| Total | 104 | 27.1 | 20 | 19.2 | 4.6421 (0.031) | 11 | 10.6 | 0.6681 (0.414) | |
| >500 | None | 208 | 83.5 | 30 | 14.4 | 18 | 8.7 | ||
| Acute | 20 | 8 | 6 | 30 | 2 | 10 | |||
| Chronic | 21 | 8.4 | 2 | 9.5 | 1 | 4.8 | |||
| Total | 249 | 64.8 | 38 | 15.3 | 0.6861 (0.408) | 21 | 8.4 | 0.0791 (0.778) | |
In correlating CD4 levels to diarrheal level and parasitic infection directly in Table 3 children with low CD4 counts (<200 cells/µL) who had no diarrhea had a higher rate of parasitic infection than those whom children with acute or chronic diarrhea but not statistically associated to IPIs. In both the mid and high range CD4 levels, those children with acute diarrhea had higher degrees of parasitosis than those with no or chronic diarrhea (50% and 30%, respectively, as compared to 15.3/14.3% and 14.4/9.5%). However, statistically significant correlation was observed in the range of 200–499 cells/µL (χ2=4.642, df=1, p=0.031).
Discussion
Cryptosporidiumspp. are recognized as intestinal parasites causing severe life-threatening diarrhoea in immunocompromised, immunosuppressed and malnourished patients worldwide.23 This study showed that not only Cryptosporidium but also other intestinal parasitic infections were common among HIV-positive children. Many studies in children and immunocompromised people suggest that the prevalence of cryptosporidiosis in developing countries is higher than in developed countries.24–26
The overall magnitude of intestinal parasitic infections in the study population of children receiving cART was 65 (16.9%), which is lower than an earlier study conducted in India 20.8%,27 Ethiopia 26.9%,28 Colombia 29%,29 Bahir Dar, Ethiopia 43.6%,30 similar region on adult ART patients 59.8%.21 Our finding is higher than a studies conducted in Uganda 2.17%,31 Bahir Dar, Ethiopia 5.8%,32 Peru 9.2%,33 Span 13.7%.34 These differences could be explained by the HIV treatment regimen of the study subjects and the study setting.
As compared to other study conducted somewhere else among HIV-infected children lower rates of parasitic infections were identified in our study for specific parasites, in Jamaica A. lumbricoides (2.4% vs 1.8%) and G. duodenalis (12.2% vs 2.6%),35 in Kenya G. duodenalis (11.1% vs 2.6%),36 in Addis Ababa G. duodenalis (7.6% vs 2.6%), E. histolytica/dispar (10% vs 2.0%), A. lumbricoides (6.3% vs1.8%), Taeniaspp. (3% vs 0.78%),37 in Nigeria A. lumbricoides (37.5% vs 1.8%).38 It is comparable with a study from Nigeria, A. lumbricoides (1.9% vs 1.8%).39 This lower incidence may be due to better laboratory testing, consistent patient follow-up, and better awareness of the patients themselves in implementing prevention and management actions against intestinal parasites in addition to anti-helminths and highly active antiretroviral therapy (HAART).
According to our findings, co-infection was observed with Cryptosporidium and G. duodenalis, E. histolytica/dispar, A. lumbricoides and Taenia spp. (1%) each which is different from previous studies.4 It is obvious that the availability of potable drinking water, reduced contact with animals, hygienic facilities and health education on sanitary practices contributed to the lower incidence of parasitic infestations.
In this study, we assessed that the possible associations of variables with Cryptosporidium and other parasitic infections. Diarrheal status was the only significant association observed for IPIs with (χ2=7.653, df=2, p=0.022), in agreement with a study from the same region conducted in 2009 on adult HIV patients40 and from Nigeria.41,42
Conclusions
The overall magnitude of intestinal parasitic infestations was 16.9% with Cryptosporidium present in half of the infections. Therefore, based on our finding, health education should be provided to the children’s parents and caregivers of HIV-infected children receiving cART on how to prevent exposure to pathogens transmitted by exposure to non-potable water and animals. Direct stool examination and modified Zeihl–Neelsen staining should be routinely performed for HIV-infected children during follow up mainly based on diarrheal status.
Limitation Of The Study
We used a convenient sampling technique in which consecutive children were involved in the study, which may explain a bias toward children above 7 years, as compared to the younger children. A single stool sample was used to detect intestinal parasites, which may lead to false negative results. Two or more independently collected stool specimens are recommended to ensure diagnostic sensitivity to detect intestinal parasitic infections.43 Only HIV-infected children were tested; there was no control group of children not infected with HIV to compare the impact of HIV-infection on parasitic infection rates.
What Is Already Known About This Topic?
HIV positive children are highly susceptible for opportunistic infections.
Cryptosporidium is a common opportunistic parasite among these patients.
Different risk factors were reported for intestinal parasites among HIV positive children other than immune status.
What This Study Adds
The study determined the magnitude of Cryptosporidium and other intestinal parasites among HIV children in the study sites.
Our study identified diarrheal status as the only statistical significant associated variable for IPIs.
Acknowledgments
We would like to thank Hawassa University, HU-CSH laboratory staff, ART clinic staff, and the study subjects, parents, and children.
We would like to thank also Mrs Sally Callihan and Dr Donald Callihan (bacteriologists) from the USA for language editing.
An abstract of this paper was presented at the International Workshop on Microbiome in HIV Pathogenesis, Prevention, and Treatment as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” Virology Education (https://virology.eventsair.com/QuickEventWebsitePortal/microbiomeinhiv2019/program/Agenda/AgendaItemDetail?id=7dc53cfc-a1a4-4cf3-a6de-106f35fd3a1b).
Funding Statement
The study was partially sponsored by Hawassa University for data collection and laboratory diagnosis.
Ethical Clearance
The Institutional Review Board of the College of Medicine and Health Sciences, Hawassa University approved the study. Involvement was voluntary and informed written consent was obtained from caregivers of the study participants. Physicians managed those found to be infected with any pathogenic intestinal parasites. This study was conducted in accordance with the declaration of Helsinki.
Availability Of Data And Material
All the raw data supporting the findings can be obtained from all authors.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Communicable Disease Control and Infection Prevention (CDC). Cryptosporidiosis: sources of infection and risk factors April; 2015. Available from: http://wwwcdcgov/parasites/crypto/infectionsourceshtml.
- 2.UNAIDS JUNPoHA.Geneva, Switzerland: Children and HIV Factsheet; Available from: https://wwwunaidsorg/sites/default/files/media_asset/FactSheet_Children_enpdf. Accessed 2016. July. [Google Scholar]
- 3.Iqbal A, Lim A, Al Mahdy M, Dixon B, Surin J. Epidemiology of cryptosporidiosis in HIV-infected individuals-a global perspective. J AIDS Clin Rep. 2012;1(9):1–16. [Google Scholar]
- 4.Abaver D, Nwobegahay J, Goon D, Iweriebor B, Anye D. Prevalence of intestinal parasitic infections among HIV/AIDS patients from two health institutions in Abuja, Nigeria. Afr Health Sci. 2011;11(3):24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark DP. New insights into human cryptosporidiosis. Clin Microbiol Rev. 1999;12(4):554–563. doi: 10.1128/CMR.12.4.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariam ZT, Abebe G, Mulu A. Opportunistic and other intestinal parasitic infections in AIDS patients, HIV seropositive healthy carriers and HIV seronegative individuals in southwest Ethiopia. East Afr J Public Health. 2008;5(3):169–173. [PubMed] [Google Scholar]
- 7.Sim S, Yu J-R, Lee Y-H, et al. Prevalence of Cryptosporidium infection among inhabitants of 2 rural areas in White Nile State, Sudan. Korean J Parasitol. 2015;53(6):745. doi: 10.3347/kjp.2015.53.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque R. Human intestinal parasites. J Health Population Nutrit Home. 2007;25(4):387–391. [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes S, Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006;28(11):577–588. doi: 10.1111/j.1365-3024.2006.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mølbak K, Andersen M, Aaby P, et al. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr. 1997;65(1):149–152. doi: 10.1093/ajcn/65.1.149 [DOI] [PubMed] [Google Scholar]
- 11.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148(5):497–506. [DOI] [PubMed] [Google Scholar]
- 12.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707 [DOI] [PubMed] [Google Scholar]
- 13.Liner J, Hall C, Robertson K. Effects of antiretroviral therapy on cognitive impairment. Curr HIV/AIDS Rep. 2008;5:64–71. doi: 10.1007/s11904-008-0011-7 [DOI] [PubMed] [Google Scholar]
- 14.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16(1):18603. doi: 10.7448/IAS.16.1.18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol. 2007;63(4):387–393. doi: 10.1111/j.1365-2125.2007.02873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leder K, Weller DPF, Weller PF, McGovern BH. Treatment and Prevention of Cryptosporidiosis. (Monografía En Internet) Walthman (MA): UpToDate; 2018. [Google Scholar]
- 17.Jahangir HossainID M, Debasish Saha MA, Dilruba Nasrin WC, et al. Cryptosporidium infection in rural Gambian children: epidemiology and risk factors. PLoS Negl Trop Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atsimbom Neville Tombang NFA, Bobga TP, Nkfusai CN, Ngandeu Mongoue Collins SBN, Diengou NH, Cumber SN. Prevalence and risk factors associated with cryptosporidiosis among children within the ages 0–5 years attending the Limbe regional hospital, southwest region, Cameroon. BMC Public Health. 2019;19:1144. doi: 10.1186/s12889-019-7484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlos Alberto Velasco FM, López P. Cryptosporidiosis in Colombian children with HIV/AIDS infection. Colomb Med. 2011;42:418–429. [Google Scholar]
- 20.World Health Organization. Diarrhoeal disease; 2017. Available from: https://wwwwhoint/news-room/fact-sheets/detail/diarrhoeal-disease.
- 21.Shimelis T, Tassachew Y, Lambiyo T. Cryptosporidium and other intestinal parasitic infections among HIV patients in southern Ethiopia: significance of improved HIV-related care. Parasit Vectors. 2016;9(1):270. doi: 10.1186/s13071-016-1554-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt F, Bergh R, Bernasconi A, et al. Using BD Vacutainer CD4 stabilization tubes for absolute cluster of differentiation Type 4 cell count measurement on BD FacsCount and Partec cyflow cytometers: a method comparison study from Zimbabwe. PLoS One. 2015;10:e0136537. doi: 10.1371/journal.pone.0136537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai N, Sarkar R, Kang G. Cryptosporidiosis: an under-recognized public health problem. Trop Parasitol. 2012;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirley D-AT, Moonah SN, Kotloff KL. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis. 2012;25(5):555–563. doi: 10.1097/QCO.0b013e328357e569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snelling W, Xiao L, Ortega-Pierres G, et al. Cryptosporidiosis in developing countries. J Infect Dev Ctries. 2007;1:242–256. doi: 10.3855/jidc.360 [DOI] [PubMed] [Google Scholar]
- 26.Shrivastava AK, Kumar S, Smith WA, Sahu PS. Revisiting the global problem of cryptosporidiosis and recommendations. Trop Parasitol. 2017;7(1):8–17. doi: 10.4103/2229-5070.202290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain S, Singh AK, Singh RP, Bajaj J, Damle AS. Spectrum of opportunistic and other parasites among HIV/AIDS patients attending a tertiary care hospital. Asian Pac J Trop Dis. 2014;4(6):480–483. doi: 10.1016/S2222-1808(14)60610-1 [DOI] [Google Scholar]
- 28.Adamu H, Petros B, Zhang G, et al. Distribution and Clinical Manifestations of Cryptosporidium Species and Subtypes in HIV/AIDS Patients in Ethiopia. PLoS Negl Trop Dis. 2014;8(4):e2831. doi: 10.1371/journal.pntd.0002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco CA, Méndez F, López P. Cryptosporidiosis in Colombian children with HIV/AIDS infection. Colombia Médica. 2011;42:418–429. [Google Scholar]
- 30.Alemu A, Shiferaw Y, Getnet G, Yalew A, Addis Z. Opportunistic and other intestinal parasites among HIV/AIDS patients attending Gambi higher clinic in Bahir Dar city, North West Ethiopia. Asian Pac J Trop Med. 2011;4(8):661–665. doi: 10.1016/S1995-7645(11)60168-5 [DOI] [PubMed] [Google Scholar]
- 31.Nakibirango J, Mugenyi V, Nsaba D, Nsimemukama A, Rugera SP, Okongo B. Prevalence of cryptosporidiosis and hygiene practices among HIV/AIDS patients in southwest Uganda. HIV/AIDS (Auckland, NZ). 2019;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiros H, Nibret E, Munshea A, Kerisew B, Adal M. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot referral Hospital, Bahir Dar, Ethiopia. Int J Infect Dis. 2015;35:80–86. doi: 10.1016/j.ijid.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 33.Cama VA, Ross JM, Crawford S, et al. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J Infect Dis. 2007;196(5):684–691. doi: 10.1086/519842 [DOI] [PubMed] [Google Scholar]
- 34.Barboni G, Candi M, Ines Villace M, Leonardelli A, Balbaryski J, Gaddi E. [Intestinal cryptosporidiosis in HIV infected children]. Medicina. 2008;68(3):213–218. [PubMed] [Google Scholar]
- 35.Barrett D, Steel-Duncan J, Christie C, Eldemire-Shearer D, Lindo J. Absence of opportunistic parasitic infestations in children living with HIV/AIDS in children’s homes in Jamaica: pilot investigations. West Indian Med J. 2008;57(3):253–256. [PubMed] [Google Scholar]
- 36.Pavlinac PB, John-Stewart GC, Naulikha JM, et al. High-risk enteric pathogens associated with HIV-infection and HIV-exposure in Kenyan children with acute diarrhea. AIDS. 2014;28(15):2287. doi: 10.1097/QAD.0000000000000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mengist HM, Taye B, Tsegaye A. Intestinal parasitosis in relation to CD4+ T cells levels and anemia among HAART initiated and HAART naive pediatric HIV patients in a model ART center in Addis Ababa, Ethiopia. PLoS ONE. 2015;10(2):e0117715. doi: 10.1371/journal.pone.0117715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orji M-LC, Onyire NB, Ibe BC, Ibekwe R. Effect of Helminth infestation in children infected with Human Immunodeficiency Virus (HIV). J Nepal Paediatr Soc. 2017;37(1):25–30. doi: 10.3126/jnps.v37i1.16474 [DOI] [Google Scholar]
- 39.Oyedeji OA, Adejuyigbe E, Oninla SO, Akindele AA, Adedokun SA, Agelebe E. Intestinal parasitoses in HIV infected children in a Nigerian Tertiary Hospital. J Clin Diagn Res. 2015;9(11):SC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assefa S, Erko B, Medhin G, Assefa Z. Shimelis T: intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect Dis. 2009;9(1):155. doi: 10.1186/1471-2334-9-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jegede EF, Oyeyi ETI, Bichi AH, Mbah HA, Torpey K. Prevalence of intestinal parasites among HIV/AIDS patients attending infectious disease Hospital Kano, Nigeria. Pan Afr Med J. 2014;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akinbo FO, Okaka C, Omoregie R. Prevalence of intestinal parasitic infections among HIV patients in Benin City, Nigeria. Libyan J Med. 2010;5(1):5506. doi: 10.3402/ljm.v5i0.5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cartwright CP. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37(8):2408–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Communicable Disease Control and Infection Prevention (CDC). Cryptosporidiosis: sources of infection and risk factors April; 2015. Available from: http://wwwcdcgov/parasites/crypto/infectionsourceshtml.
Data Availability Statement
All the raw data supporting the findings can be obtained from all authors.