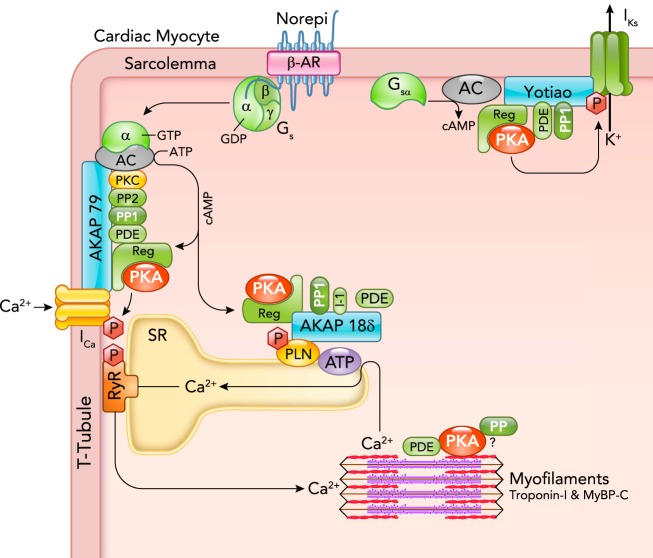
FIGURE 1.
Cardiac myocyte β-AR, cAMP, and PKA signaling proteins are associated with AKAPs
A β-AR activated by norepinephrine (Norepi) activates a GTPase protein (Gs) whose α-subunit activates adenylyl cyclase (AC) to produce cAMP from ATP. That cAMP binds to the regulatory subunit of PKA (Reg) to activate the catalytic subunit (shown as PKA) by relaxing the interaction with Reg. That active PKA can selectively phosphorylate nearby target proteins including sarcolemmal L-type Ca channels, which increases ICa, SR phospholamban (PLN), which increases SR Ca-ATPase activity, and KCNQ1 to enhance delayed rectifier K+ current IKs. PKA is anchored at these three targets, respectively, by AKAP79 (in human or mouse AKAP150; made by AKAP5 gene), AKAP18δ (made by AKAP7 gene), and Yotiao (made by AKAP9 gene). These AKAPs also bind additional cAMP-PKA modulators (PP1, PP2, PDEs, PKC). Troponin T has been proposed to be an AKAP (56), and TPNI and MyBP-C phosphorylation are regulated by PKA, PPs, and PDEs, but those mechanisms are less well resolved. See Ref. 16 for a comprehensive AKAP review.