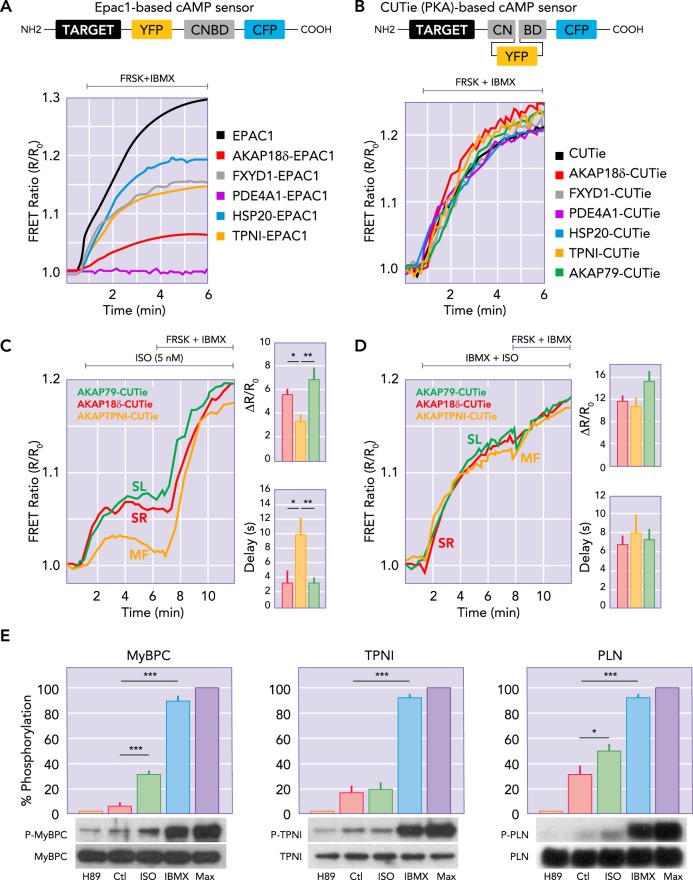
FIGURE 2.
Targeted FRET-based reporters provide direct measurement of [cAMP] at discrete molecular sites in myocytes
The more traditional cAMP reporter (A) has the cAMP binding domain (CNBD) between CFP and YFP, with the targeting domain (TARGET) attached to the NH2 terminal. The newly designed CUTie reporters (B) with different protein arrangement improves the consistency of the reporter affinity and dynamic range, regardless of targeting location (curves in B vs. A). In adult rat ventricular myocytes, targeted CUTies (C) indicated faster initial rise times and larger [cAMP] increases at the SL and SR vs. MF in response to 5 nM isoproterenol (ISO) exposure. When PDE were inhibited and AC was directly activated by forskolin, all three sensors gave the same signal (D), indicating that the nanodomain differences in [cAMP] induced by ISO were due in large part to the presence of PDE. E: PKA-dependent phosphorylation of MyBPC, TNI, and PLN at baseline (Ctl) in response to 0.3 nM ISO with minimum and maximum phosphorylation assessed by treatment with H89 (PKA inhibitor) or IBMX (or IBMX+forskolin; Max). Panels were redrawn based on parts of Figs. 1, 3, and 5 in Ref. 57, with permission from Nature Communications.