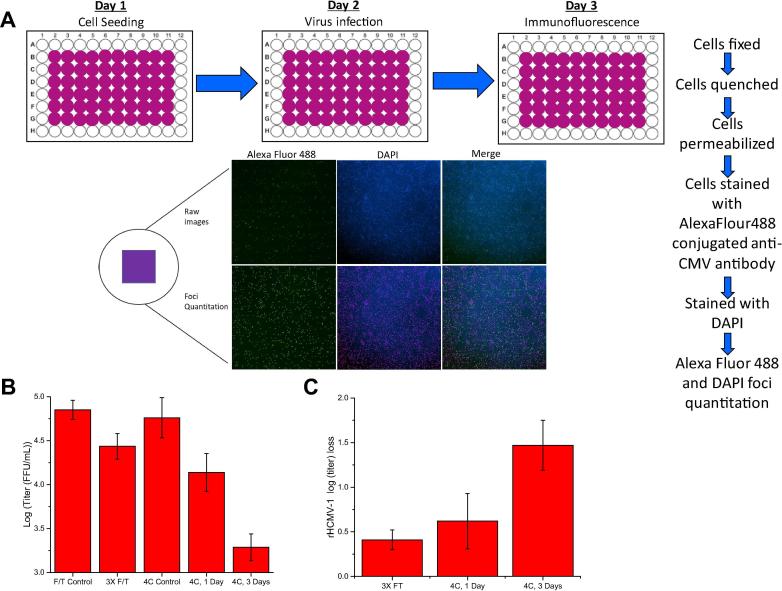
Fig. 1.
Overview of viral infectivity assay (immediate-early fluorescence focus assay, IE-IFA) and rHCMV-1 titer losses after freeze-thaw cycling and short-term storage at 4 °C in TNS buffer. (A) Schematic of the IE-IFA assay including immunofluorescence readout to determine number of infectious viral particles. (B) log (titer) values of rHCMV-1 control and stressed samples in TNS buffer (see text), and (C) log loss of rHCMV-1 viral titer (compared to control) after three freeze-thaw cycles from −80 °C or incubation at 4 °C for 1 or 3 days. Log loss was calculated by subtracting the log (titer) value of the control sample (viral vector stock in TNS buffer diluted 1:150 in TNS buffer and stored at −80 °C) from the log (titer) value of the stressed sample prepared in same manner. Stability data are an average of 12 measurements, except for the 4 °C, 3 day timepoint which was an average of 6 measurements, with the error bars representing the standard deviation.