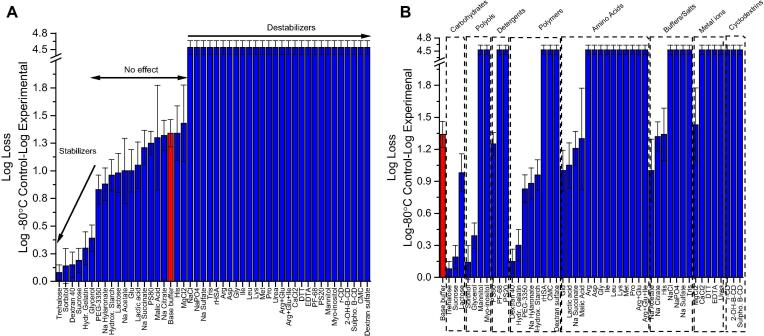
Fig. 2.
Log loss of rHCMV-1 viral infectivity titer after 3 freeze-thaw cycles from −80 °C in the presence of 48 different excipients. (A) Excipients listed in order of protective effect on viral vector stability, and (B) excipients listed by class of additives. The rHCMV-1 stock was diluted 1:150 into each excipient containing solution in a base buffer (10 mM Histidine, pH 6.5) in polypropylene microcentrifuge tubes, subjected to 3 freeze-thaw cycles and analyzed by IE-IFA. Log loss of viral titer was calculated by subtracting the log (titer) of the control sample, which was rHCMV-1 bulk diluted 1:150 in TNS buffer and stored at −80 °C, from the log(titer) of the experimental formulations after 3 freeze-thaw cycles. Stability data are an average of three measurements and the error bars represent the standard deviation. The viral vector in base buffer alone (10 mM Histidine, pH 6.5) is indicated by the red colored bar.