Figure 7.
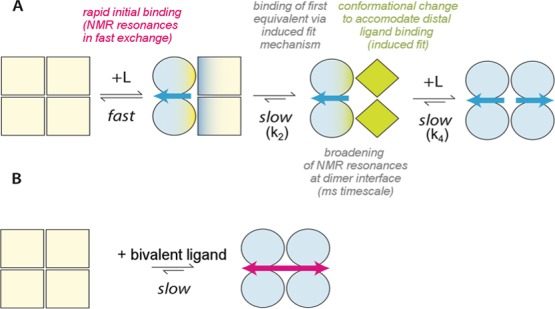
Scheme of the binding of tafamidis and mds84 by TTR. (A) Binding of tafamidis. Apo-TTR, which is a symmetrical homotetramer is shown as pale-yellow squares. Binding of a single molecule of tafamidis initially induces changes in the occupied half molecule dimer, shown here as light-blue green circles, and is then followed by conformational changes in the unoccupied dimer, shown as green diamonds. When the second tafamidis molecule is bound, the TTR tetramer becomes symmetrical again, with the protomers illustrated as light blue circles. The off-rates measured by SPR, k2 = 0.008 Hz and k4 = 0.06 Hz, are consistent with global slow exchange in which the slower k4 is responsible for the negative cooperative effect of binding of this monovalent ligand. (B) Binding of mds84 induces symmetrical changes into the holo-TTR shown as light blue circles.
