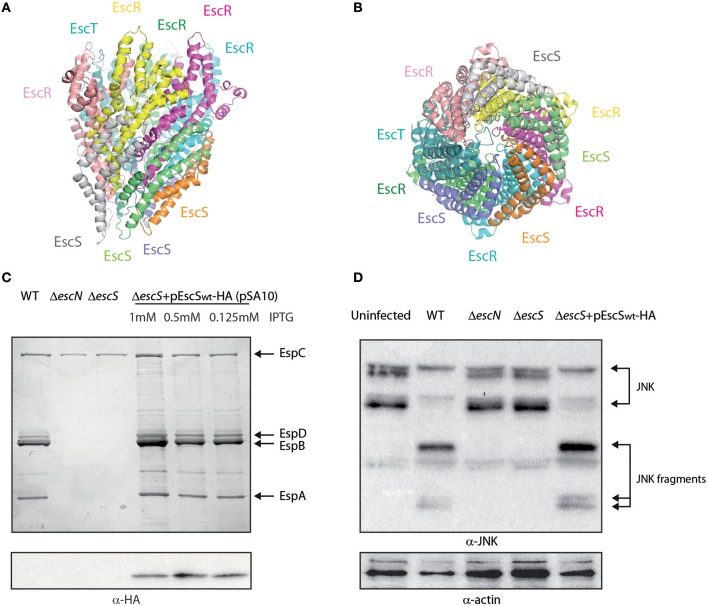
Figure 1.
EscS can complement ΔescS. (A) Side-view of the EscR5S4T complex based on the PDB 6F2D complex of the flagellar FliP5Q4R complex of Salmonella. Protein subunits are labeled with their corresponding color in the model. (B) Bottom-view of the EscR5S4T complex. (C) Protein secretion profiles of EPEC strains grown under T3SS-inducing conditions: wild-type (WT) EPEC, ΔescN (a T3SS ATPase mutant), ΔescS, and ΔescS carrying the pEscSwt-HA and treated with different IPTG concentrations. The secreted fractions were concentrated from the supernatants of bacterial cultures and analyzed by SDS-PAGE and Coomassie blue staining. The T3SS-secreted translocators EspA, EspB, and EspD are marked on the right of the gel. Also indicated is the location of EspC, which is not secreted via the T3SS. For the ΔescN and ΔescS strains, no T3SS activity was observed. The ΔescS strain carrying the plasmid encoding EscSwt-HA showed proper T3SS activity, regardless of the IPTG concentration used. EscSwt-HA expression was detected when bacterial pellets were analyzed on SDS-PAGE and western blot analysis with an anti-HA antibody. (D) Proteins extracted from HeLa cells infected with WT, ΔescN, ΔescS, and ΔescS carrying the pEscSwt-HA were subjected to western blot analysis using anti-JNK and anti-actin (loading control) antibodies. JNK and its degradation fragments are indicated at the right of the gel. WT EPEC showed massive degradation of JNK relative to the uninfected sample and the samples infected with ΔescN or ΔescS mutant strains. EPEC ΔescS complemented with pEscSwt-HA showed similar JNK degradation profile as observed for WT EPEC, indicating a functional complementation.