Figure 2.
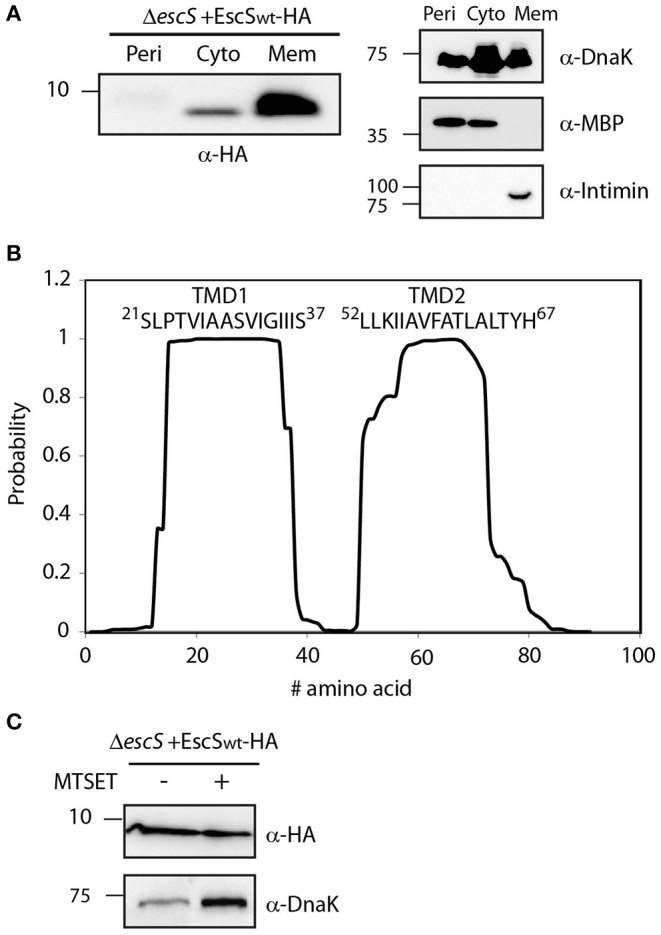
EscS localizes to the membrane fraction and its N-terminal cysteine residue is inaccessible. (A) ΔescS EPEC carrying the EscSwt-HA vector were grown under T3S-inducing conditions and fractionated into periplasmic (Peri), cytoplasmic (Cyto), and membrane (Mem) fractions. The samples were separated on an SDS-PAGE and analyzed by western blotting using anti-HA antibody. To confirm correct bacterial fractionation, the western blots were probed with anti-DnaK (cytoplasmic marker), anti-MBP (periplasmic marker), and anti-intimin (membrane marker) antibodies. (B) Analysis of the EscS sequence to rank the probability of each amino acid to be localized within the membrane, using the prediction software TMHMM (Krogh et al., 2001). Two distinct TMDs (TMD1 and TMD2) were identified. The sequences of the core TMDs are presented. (C) Spheroplasts of EPEC carrying the EscSwt-HA were grown under T3SS-inducing conditions and treated with (+) or without (–) the blocking reagent MTSET. Unblocked cysteine residues were labeled with MTSEA-biotin and biotinylated proteins were recovered with streptavidin-sepharose resin and analyzed by SDS-PAGE and western blot analysis using anti-HA antibody. The samples were also analyzed using an anti-DnaK antibody as a control for inaccessible protein.
