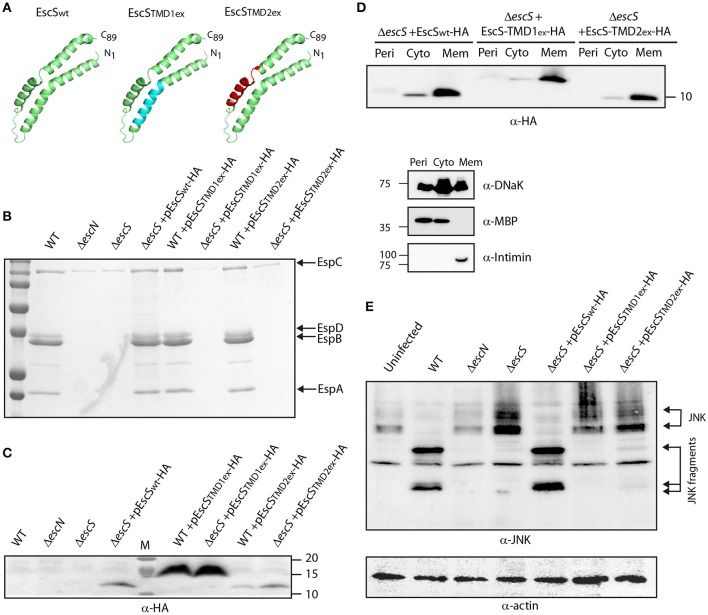
Figure 5.
Replacement of EscS TMDs by an alternative hydrophobic sequence abolishes T3SS activity. (A) 3D structure of EscSwt monomeric subunit within the EscR5S4T complex (green). The hydrophobic regions, TMD1ex and TMD2ex, that were replaced to 7L9A are marked in cyan and red, respectively. (B) Protein secretion profiles of EPEC WT, ΔescN, ΔescS, and ΔescS or WT strains carrying the pEscSwt-HA, pEscS-TMD1ex-HA or pEscS-TMD2ex-HA plasmids grown under T3SS-inducing conditions. The secreted fractions were concentrated and analyzed by SDS-PAGE and Coomassie staining. The secreted translocator EspA, EspB, and EspD are marked on the right of the gel. Also, indicated is the location of EspC, which is not secreted via the T3SS. The ΔescS strain carrying the plasmid encoding EscSwt-HA showed proper T3SS activity while replacement of either TMD1 or TMD2 to an alternative hydrophobic sequence resulted in non-functional T3SS. Expression of pEscS-TMD1ex-HA or pEscS-TMD2ex-HA within the WT EPEC strain had no dominant-negative effect on the T3SS activity. (C) EscSwt-HA, EscS-TMD1ex-HA and EscS-TMD2ex-HA expression was confirmed in the bacterial pellets obtained in (B) by SDS-PAGE and western blot analysis with an anti-HA antibody. EscS-TMD1ex-HA migrated slightly slower than EscSwt-HA and EscS-TMD2ex-HA. (D) EPEC ΔescS carrying either pEscSwt-HA, pEscS-TMD1ex-HA, or pEscS-TMD2ex-HA, were grown under T3SS-inducing conditions, fractionated into periplasmic (Peri), cytoplasmic (Cyto), and membrane (Mem) fractions and analyzed by western blot analysis with an anti-HA antibody. To confirm correct bacterial fractionation, the western blots were probed with anti-DnaK (cytoplasmic marker), anti-MBP (periplasmic marker), and anti-intimin (membrane marker) antibodies. (E) Proteins extracted from of HeLa cells infected with WT, ΔescN, ΔescS, or ΔescS carrying the pEscSwt-HA, pEscS-TMD1ex-HA or pEscS-TMD2ex-HA, were subjected to western blot analysis using anti-JNK antibody and anti-actin (loading control). JNK and its degradation fragments are indicated. WT EPEC showed massive degradation of JNK relative to the uninfected sample and the samples infected with ΔescN or ΔescS mutant strains. EPEC ΔescS complemented with pEscSwt-HA showed a JNK degradation profile similar to that of WT EPEC, indicating a functional complementation, while the ΔescS transformed with EscS exchanged TMDs (ΔescS+ pEscS-TMD1ex-HA or ΔescS+ pEscS-TMD2ex-HA) vectors showed a JNK profile similar to that of the uninfected sample.