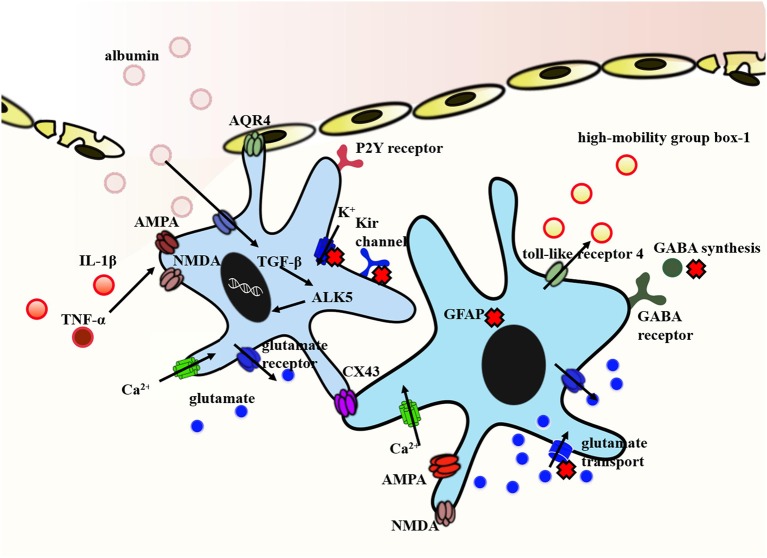
Figure 2.
Representative molecular mechanism of post-traumatic epilepsy involved in astrocytes. The exposure of brain tissue to extravasated serum albumin induces activation of TGF-β/ALK5 signaling in astrocytes. IL-1β and TNFα implicated in NMDA and AMPA receptor activation inducing Ca2+ influx in a dose-dependent manner. In addition, IL-1β and TNFα down-regulate the expression of Kir channels and weaken its clearance of extracellular K+. Decreased expression of Kir channels in concert with dislocation of AQP4 channels in astrocytes contribute to impaired K+ buffering. In this context, reactive astrocytes dramatically transform in their morphology and function. Reactive astrocyte is characterized by the proliferation of astrocytes and the hallmark accumulation of GFAP. Traumatic brain injury also causes a deranged glutamate uptake and a decreased GABA release. The high-mobility group box-1 is an additional inflammatory agent produced by reactive astrocytes and mediated by the toll-like receptor 4. Finally, the expression of Cx43 protein is different in human and animal. It is generally shown to be increased in human, whereas findings from animal models are conflicting. Arrows show the flow direction. The red forks represent the disordered functions. Some mechanisms are indicated by abbreviations. GFAP, glial fibrillary acidic protein; NMDA, N-methyl-D-aspartate; AMPA, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA, γ-aminobutyric acid; AQP4, aquaporin-4; TGF-β, transforming growth factor-β; Cx43, connexin-43; IL-1β, interleukin-1beta.