Figure 3.
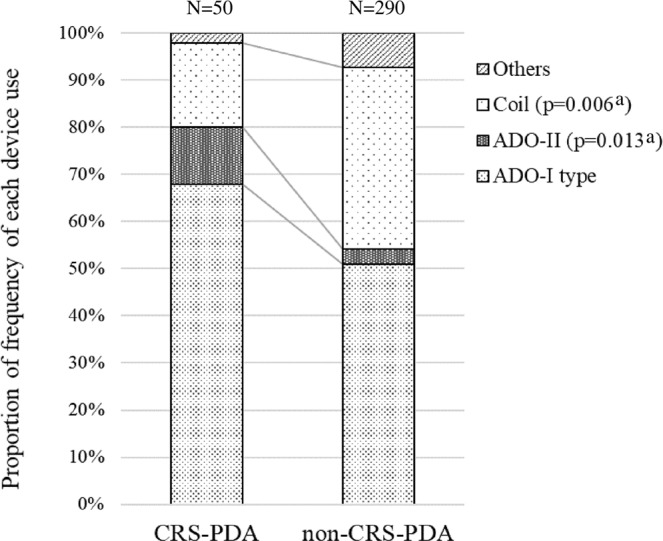
Proportion of frequency of each device use for patent ductus arteriosus occlusion in CRS-PDA and non-CRS-PDA. (a) Fischer’s exact test CRS-PDA; children with congenital rubella syndrome and patent ductus arteriosus treated by transcatheter closure, non-CRS-PDA; children without congenital rubella syndrome and with patent ductus arteriosus treated by transcatheter closure, ADO-I type; PDA occluders with retention skirt, ADO-II; Amplatzer™ Duct Occluder II, Others; other occluders including muscular ventricular septal defect occluder, atrial septal defect occluder, coil for ventricular septal defect, and use ADO-II and coil in combination.
