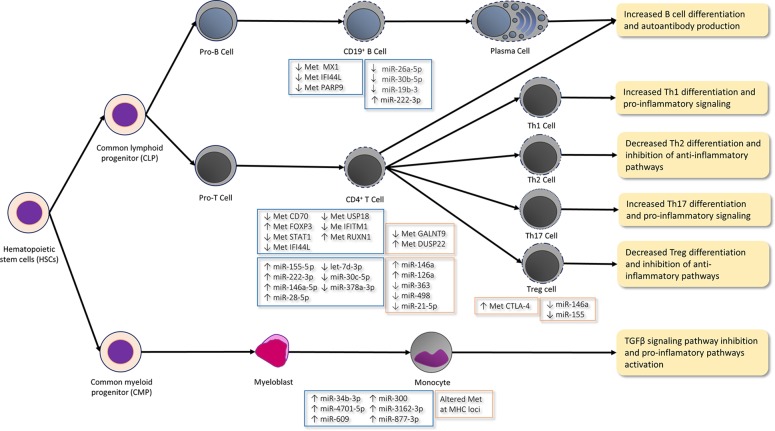
Figure 1.
Epigenetic mechanisms in immune cells differentiation and function in arthritis rheumatoid (AR; orange boxes) and Sjögren syndrome (SS; blue boxes). In the CD19+ B-cells of SS patients, type I IFN genes, such as genes MX1, IFI44L, and PARP9, are hypomethylated (↓ Met), which agrees with the hypomethylation of some other IFN-γ-regulated genes (e.g. STAT1, IFI44L, USP18, and IFITM1) in CD4+ T-cells and with the IFN response activation observed in these patients. Moreover, low levels (↓) of miR-26a-5p, miR-30b-5p, and miR-19b-3p and high miR-222-3p levels (↑) are reported in the CD19+ B-cells of SS patients. Low levels miR-30b-5p bring about an increase of the B-cell activating factor (BAFF) and autoantibody production. The hypomethylation of the CD70 promoter in the CD4+ T-cells of SS patients leads to the increased expression of the CD70 gene, which interacts with CD27 during B- and T-cell contact by promoting plasma cell differentiation and IgG production. The hypermethylation (↑ Met) of the RUNX1 gene in SS regulates the maturation of hematopoietic stem cells. The high expression of inflamma-miRs miR-155-5 p, miR-222-3p, and miR-146a-5p and the low expression of let-7d-3p, miR-30c-5p, and miR-378a-3p are described in CD4+ T-cells. ImmflamamiRs miR-155 and mir-146a inhibit Th2 proliferation and promote the Th1 response. Furthermore, miR-155 also promotes the differentiation and function of Th17 and Treg. The hypermethylation of the FOXP3 promoter lowers the FOXP3 expression in the CD4+ T-cells of SS and is characteristic of the differentiated and functional Treg. The hypermethylation of the DUSP22 gene and the hypomethylation of the GALNT9 gene in CD4+ T-cells are implicated in the IL-6/STAT3-mediated signaling pathway by contributing to autoimmunity and promoting the pro-inflammatory Th17 cells differentiation in RA patients. High inflamma-miR miRNA-146a levels and low miR-363, miR-21-5, and miR-498 levels are reported in the CD4+ T-cells of RA patients. Low miR-21-5p levels promote Th17 cell differentiation, while suppressing Treg development. Furthermore, high miR-126a levels inhibit DNM1I which, in turn, produces the hypomethylation of CD11a and CD70 by increasing their expression and promoting the autoimmune response. The methylation of the CTLA4 promoter inhibits Treg activity in RA. In contrast to the miRNA levels observed in the CD4+ T-cells of RA patients, miR-146a and miR-155 are lower in Treg cells after T-cell stimulation. miR-146a regulates NF-kß signaling and targets STAT1 by thus controlling the Treg function. Hence, the low levels of this miRNA bring about the inhibition of Treg differentiation and function. The differential methylated sites located in the MHC region are described in the CD14+ monocytes of RA patients. Moreover, the levels of miR-34b-3p, miR-4701-5p, miR-609, miR-300, miR-3162-3p, and miR-877-3p are higher in the monocytes of SS patients, which may inhibit the TGFβ signaling pathway. Thus, an unbalanced differentiation takes place from the CD4+ T-cells to the Th1 and Th17 cells, which promotes pro-inflammatory pathways and increases autoantibody production in both diseases.