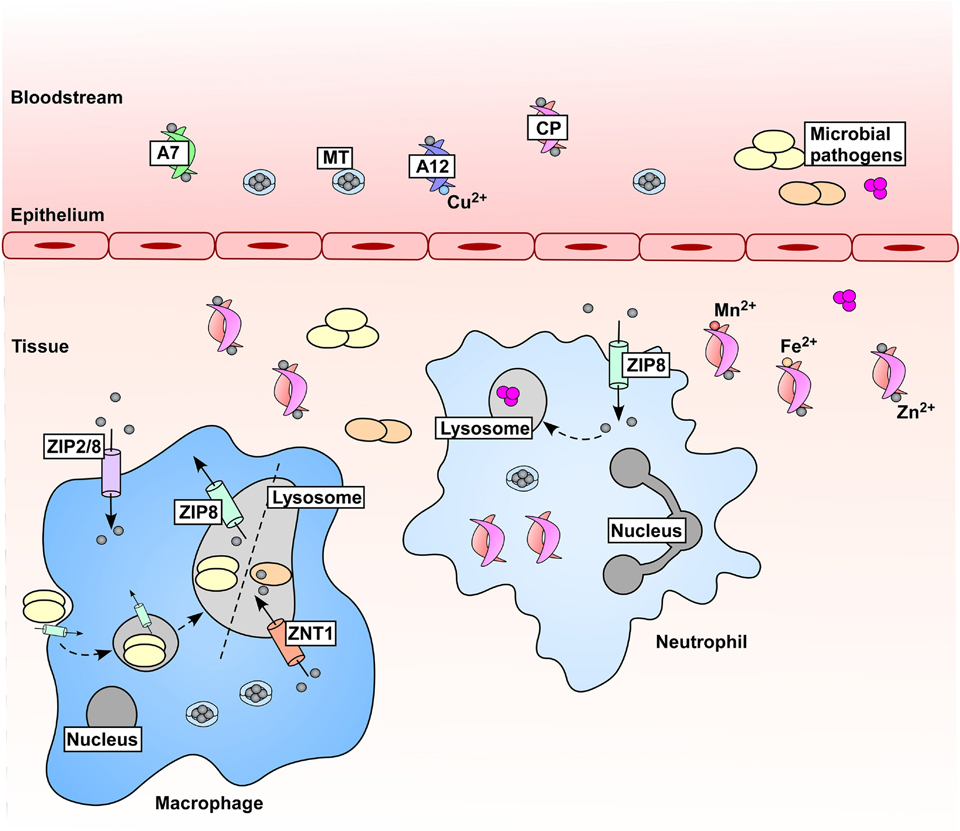
Figure 2.
Zn is mobilized in hosts during microbial infections and inflammation. In response to microbial challenge, serum Zn drops through increased expression of zinc transporters (not depicted) and mobilization and uptake of Zn-binding metallothioneins (MTs). Immune cells contribute to changes in Zn availability, where expression of ZIP Zn importers drives accumulation of Zn within these cells. Zn is also mobilized within immune cells; Mycobacterium tuberculosis (orange ovals) experiences Zn intoxication within macrophages in a ZNT1-dependent manner, and Streptococcus pyogenes (pink spheres) is poisoned by Zn within the neutrophil lysosome [69, 70]. Conversely, the fungal pathogen Histoplasma capsulatum (yellow ovals) has been shown to be Zn-starved within the macrophage lysosome [71]. These two mechanisms (Zn intoxication and Zn starvation) within the macrophage lysosome are denoted by a dashed line. Additionally, metal-chelating proteins are produced as part of the inflammatory response to further reduce Zn availability for pathogens, including those within the S100 protein family. S100A7 (A7) binds Zn and is produced in high abundance by keratinocytes, and S100A12 (A12) binds both Zn and Cu. The heterodimer of S100A8/S100A9, known as calprotectin (CP) [159], binds Zn with high affinity, as well as other divalent cations. CP is a major component of the neutrophil cytoplasmic protein content and is abundant at sites of infection and inflammation within vertebrates.