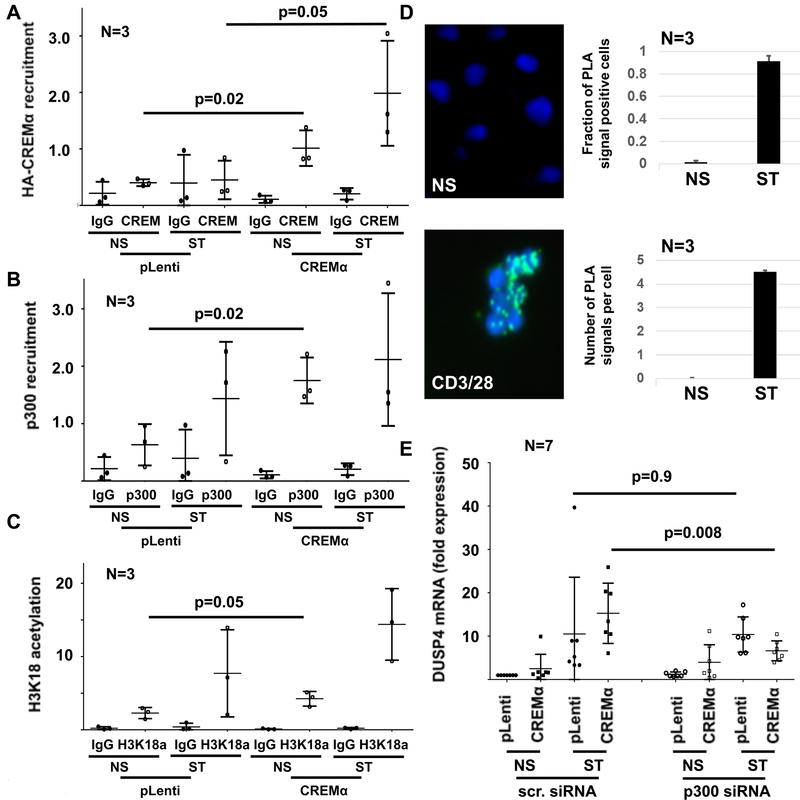
Figure 4: CREMα recruits p300 to the DUSP4 promoter.
A) Overexpression of HA-CREMα results in recruitment to the DUSP4 promoter as assessed by ChIP (NS: not stimulated; ST: stimulated with plate-bound andi-CD3 and anti-CD28 antibodies). B) The transcriptional co-activator p300 recruits to the same region in the DUSP4 promoter. C) Since p300 acts as histone acetyl transferase that can mediate H3K18 acetylation, this epigenetic modification was tested in the same region. CREMα overexpressing cells exhibit increased H3K18ac at the DUSP4 promoter. D) Proximity ligation assays (PLA) indicate interactions between CREMα and p300 in response to stimulation of primary human CD4+ T cells with plate-bound anti-CD3 and anti-CD28 antibodies. Displayed are results from 3 independent experiments representing a total of 246 unstimulated (NS) and 86 stimulated (ST) cells. The left panel shows PLA signals (green) in NS and CD3/CD28 ST CD4+ T cells as indicated; the right panel displays the fraction of PLA signal positive out of all CD4+ T cells (top) and the median number of signals per cell (bottom). E) Knock-down of p300 in CREMα overexpressing cells resulted in reduced mRNA expression of DUSP4 when compared to controls (untransfected or transfected with scrambled control siRNA; scr. siRNA) suggesting functional interactions between CREMα and p300 (qRT-PCR). In all figures mean values and standard deviations are displayed.