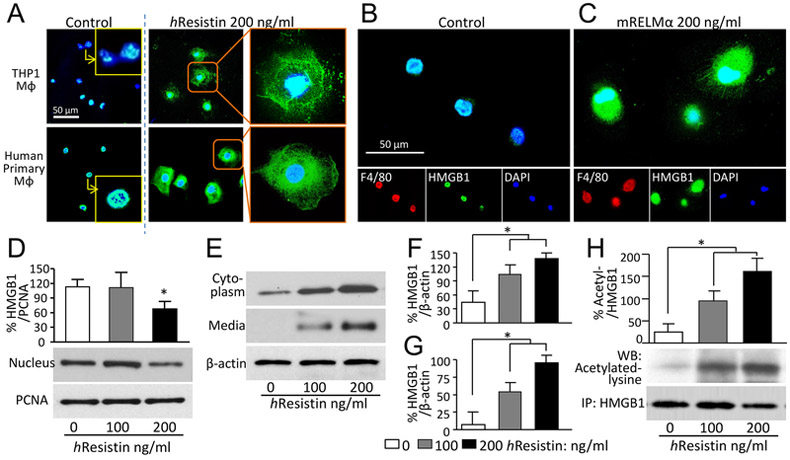
Figure 3.
RELMα/hResistin induces acetylation, nucleus-to-cytoplasm translocation, and secretion of HMGB1 in macrophages. (A) THP-1-differentiated macrophages (upper panels) and human primary macrophages differentiated from CD14+ peripheral blood monocytes (lower panels) were treated with hResistin and immunofluorescently stained with anti-HMGB1 Abs (green). n = 6. Magnification: 400X. Higher magnification images in the insets show control vehicle-treated macrophages with HMGB1 nuclear retention. MΦ, macrophages. (B, C) Mouse macrophages collected from bronchoalveolar lavage were treated with control medium (B) or lab-made recombinant mouse (m) RELMα protein (C). The RELMα-facilitated HMGB1 intracellular mobilization was determined by immunocytochemistry with anti-F4/80 (red) and anti-HMGB1 (green) Abs. Representative results from three independent experiments with 4-6 animals in each group are shown. Magnification: 400X. (D) Western blot analysis of nuclear HMGB1 expression in hResistin-treated THP-1-differentiated human macrophages. Proliferating cell nuclear antigen (PCNA), a nuclear housekeeping protein, served as a control. Data represent means ± SEM (n = 3). *p < 0.05 vs control medium. (E) Cytoplasmic proteins extracted from THP-1-differentiated macrophages, as well as secreted proteins in concentrated medium, were subjected to western blotting with anti-HMGB1 Abs. As a constitutively expressed housekeeping protein, β-actin was used as a loading control. (F, G) Quantitative analysis of data in (E) for HMGB1 expression in cytoplasm (F) and in concentrated medium (G). Data are presented as means ± SEM (n = 3-4). *p < 0.05. (H) THP-1-differentiated macrophages were treated with hResistin and subjected to immunoprecipitation (IP) with anti-HMGB1 Abs. The HMGB1 acetylation was measured by using specific Abs against acetylated lysine. Data are presented as means ± SEM (n = 6). *p < 0.05.