Table 4.
In vitro activities against hMAO subtypes of coumarin derivatives.
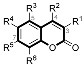
| Compounds | R1 | R2 | R4 | R5 | R6 | MAO-A a | MAO-B a | SI b |
|---|---|---|---|---|---|---|---|---|
| M29 | H | CH3 | H |
|
CH3 | 2.29 | 100 | −1.64 |
| M31 | H | (CH2)2CH3 | H |
|
H | 0.50 | 64.57 | −2.11 |
| M32 | CH3 | CH3 | Cl |
|
H | 81.28 | 0.60 | 2.13 |
| M43(7a) | H | CH3 | H |
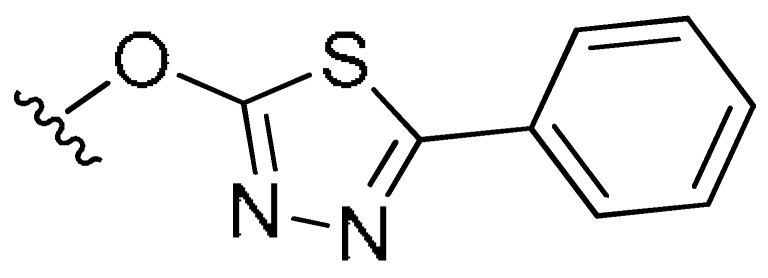
|
H | 0.16 | 0.36 | −0.35 |
| FR1 | –(CH2)3– | H |
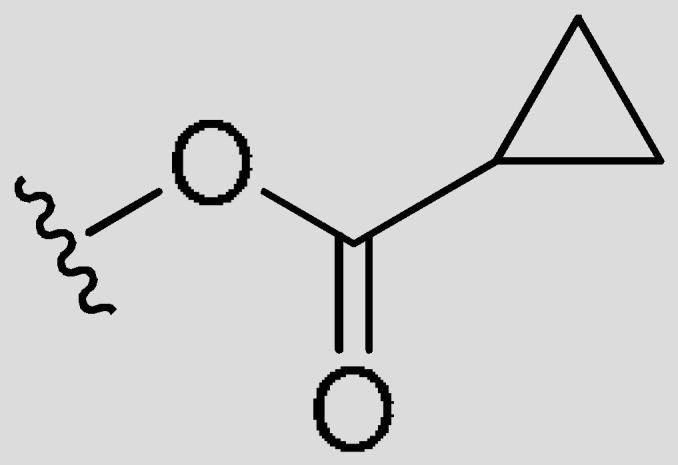
|
CH3 | 0.0015 | 75% (>1 uM) | <−2.82 | |
| FR2 | –(CH2)3– | H |
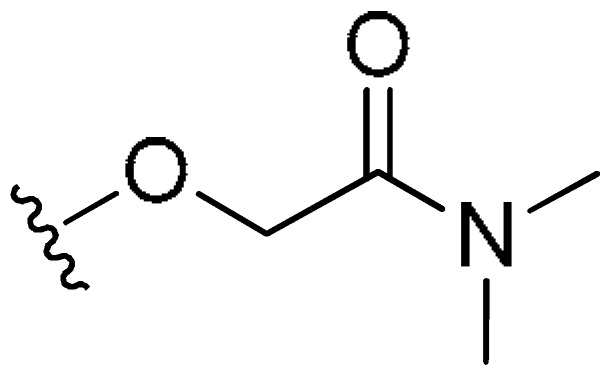
|
CH3 | 2.82 | 12% (>10 uM) | <−0.55 | |
| FR3 | –(CH2)4– | H |
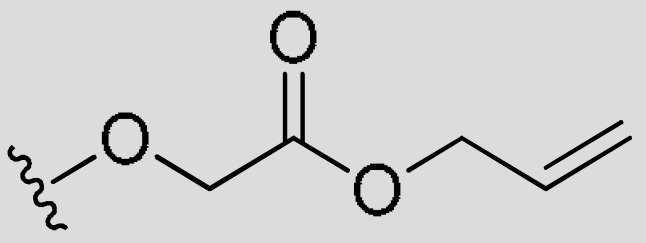
|
CH3 | 0.74 | 66% (>5 uM) | <−0.83 | |
| FR4 | –(CH2)3– | H |
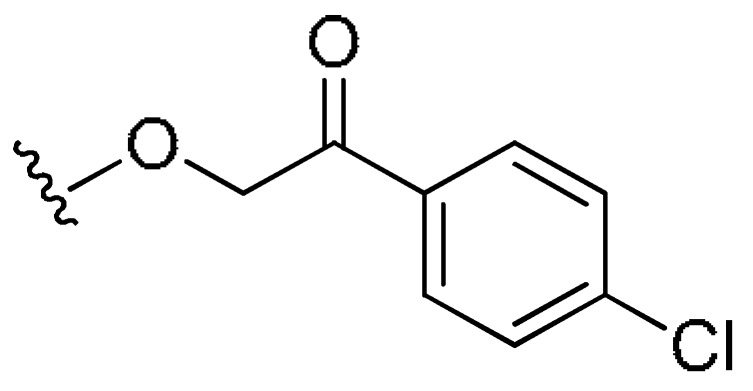
|
H | 23% (>10 uM) | 0.018 | >2.74 | |
| FR5 | H | CH3 | H |
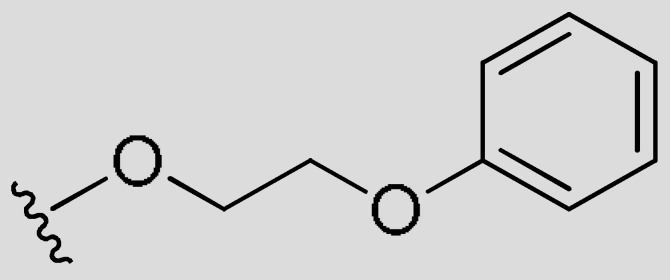
|
H | 28% (>10 uM) | 0.015 | >2.82 |
| SP1 | H | CH3 | H |
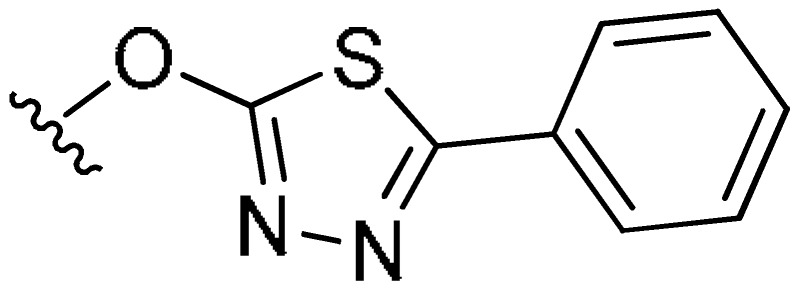
|
CH3 | 0.019 | 64% (>5 uM) | <−2.42 |
| 5d | –(CH2)3– | H |
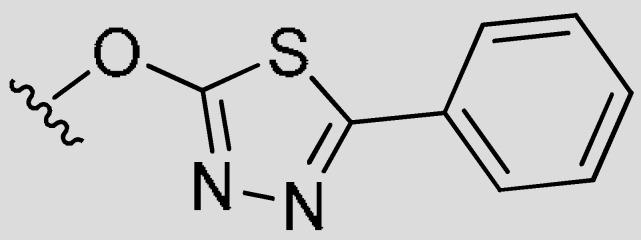
|
CH3 | 0.096 | 2.30 | −1.38 | |
| 7b | H | (CH2)2CH3 | H |
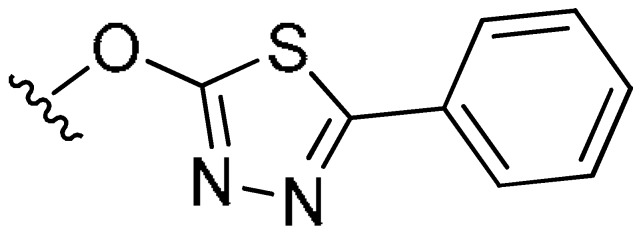
|
H | 0.024 | 0.01 | 0.38 |
| 7k | H | (CH2)2CH3 | H |
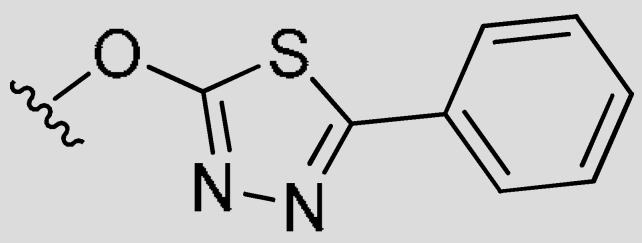
|
CH3 | 0.021 | 0.12 | −0.76 |
| 7c | CH3 | CH3 | Cl |
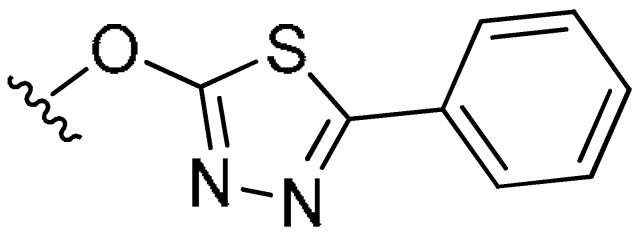
|
H | 0.81 | 0.44 | 0.27 |
| 7g | H |

|
H |
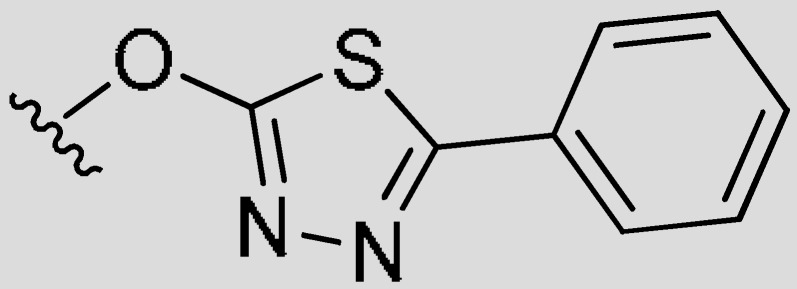
|
H | 2.00 | 6.20 | −0.49 |
a: IC50 values (μM) or inhibition activity at 10 μM against hMAO-A and hMAO-B. b: SI is the selectivity index expressed as pIC50(MAO-B)–pIC50(MAO-A).
